Could AstraZeneca’s patent be facing a significant legal challenge? Patent US12178816B2 is currently at the center of multiple lawsuits involving AstraZeneca Pharmaceuticals LP and companies such as Natco Pharma, Sandoz, Cipla, and Zydus Pharmaceuticals. This litigation involves five plaintiffs, two defendants, and two accused products, making it one of the most significant patent disputes in the pharmaceutical industry today. This extensive litigation underscores the high stakes in the pharmaceutical industry, where patent validity can make or break market exclusivity.
The patent in question pertains to an immediate-release pharmaceutical formulation of 4-[3-(4-cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluoro-benzyl]-2H-phthalazin-1-one, a compound integral to certain cancer treatments. AstraZeneca asserts that this patent safeguards its innovative formulation, while defendants argue that related patents or publications render it invalid, potentially paving the way for generic alternatives.
Identifying relevant related patents is crucial in such disputes. The Global Patent Search (GPS) tool aids legal teams by mapping key features of patents like US12178816B2 against existing patents, facilitating the discovery of pertinent related patents. This strategic insight can be pivotal in challenging or defending patent validity.
Now, let’s delve deeper into the specifics of this patent and its significance in the ongoing legal battles.
Understanding Patent US12178816B2
Patent US12178816B2 relates to an oral pharmaceutical formulation designed to enhance the solubility and bioavailability of a poorly water-soluble active ingredient. The invention specifically addresses challenges associated with formulating drugs that have low aqueous solubility, thereby improving their therapeutic efficacy. The patent outlines a method for stabilizing and delivering the active ingredient in a way that enhances its absorption in the gastrointestinal tract.
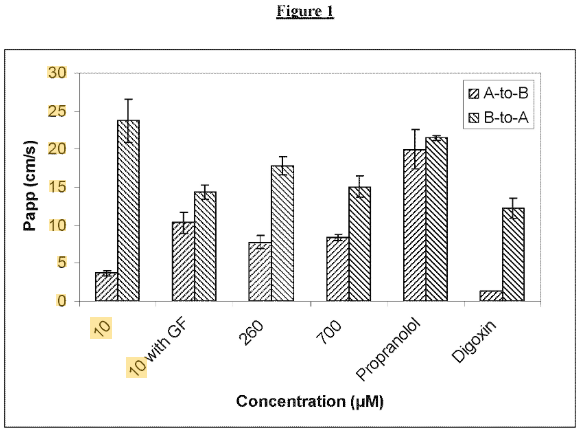
Source: Google Patents
Its four key features are:
#1. Use of solid dispersion technology – The formulation employs a solid dispersion system where the active pharmaceutical ingredient (API) is dispersed in a carrier matrix to improve solubility.
#2. Inclusion of polymeric stabilizers – Specific polymers are used to maintain the stability of the dispersion and prevent recrystallization of the API.
#3. Controlled-release mechanism – The formulation incorporates excipients that facilitate sustained or controlled release of the drug, optimizing its bioavailability.
#4. Enhanced gastrointestinal absorption – By increasing the solubility of the API, the formulation ensures more efficient absorption in the gastrointestinal tract, leading to improved therapeutic effects.
This patent is under litigation due to potential challenges related to prior art and existing formulations that may already disclose similar solubility-enhancing technologies. The key legal question is whether these techniques are novel or if prior patents and publications already describe similar methods. Establishing related patents that fully or partially discloses these features could impact the enforceability of this patent in legal proceedings.
Related Patent Reference for US12178816B2
#1. RU2440119C2
This patent, filed on September 21, 2006, describes oral solid pharmaceutical forms with a fast-release active substance. It details a pharmaceutical composition in the form of an immediate-release tablet that includes an extrudate with an active compound in an amorphous or metastable crystalline modification. The composition incorporates various polymers, glidants, and excipients to facilitate stability and bioavailability.
Key Features of this Related Patent:
- Immediate-release tablet formulation – The reference explicitly describes solid, orally applicable pharmaceutical dosage forms with immediate-release properties.
- Extrudate containing active compound – The system includes an extrudate with amorphous or metastable crystalline active compounds, supporting rapid dissolution.
- Use of polymeric carriers – The formulation contains various polymers such as hydroxypropyl cellulose (HPC), polyvinylpyrrolidone (PVP), and others, ensuring proper dispersion and stability.
- Inclusion of excipients and glidants – The composition mentions fillers, flow control agents, and other excipients to enhance processing and dissolution.
Which features of US12178816B2 are disclosed by RU2440119C2?
| Key Feature of Claim 1 | Disclosure Status |
| The pharmaceutical composition is in the form of an immediate-release tablet | Fully Disclosed |
| The composition contains an extrudate comprising 100-200 mg of 4-[3-(4-cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one (Compound 1) | Partially Disclosed |
| The extrudate contains at least one polymer selected from copovidone, povidone, hypromellose phthalate, hypromellose acetate succinate, 2-hydroxypropyl-β-cyclodextrin, hypromellose, polymethacrylates, hydroxypropyl cellulose, and cellulose acetate phthalate | Fully Disclosed |
| The extrudate contains at least one glidant | Partially Disclosed |
| The composition contains at least one excipient | Fully Disclosed |
| The weight ratio of Compound 1 to the polymer in the extrudate is from 1:1 to 1:9 | Partially Disclosed |
| The total concentration of Compound 1 in the tablet is from 10% to 35% by weight | Partially Disclosed |
Key Excerpt from RU2440119C2:
“The subject of this invention are the solid, orally applicable pharmaceutical dosage forms with immediate release of active substances containing (I), characterized in that they contain the active substance (I) in amorphous form… A method of extrusion melting to obtain the active compound (I) in amorphous form or metastable crystalline modification is preferably carried out in the presence of a polymer such as, for example, polyvinylpyrrolidone (PVP), providing enhanced dissolution and bioavailability.“
#2. US20140163071A1
This patent, filed on July 20, 2012, describes bioavailable compositions of amorphous piperidinyl compounds. It focuses on pharmaceutical compositions containing amorphous solid dispersions for enhanced solubility and bioavailability. This reference aligns with US12178816B2, particularly in its approach to stabilizing amorphous drug forms using polymeric carriers.
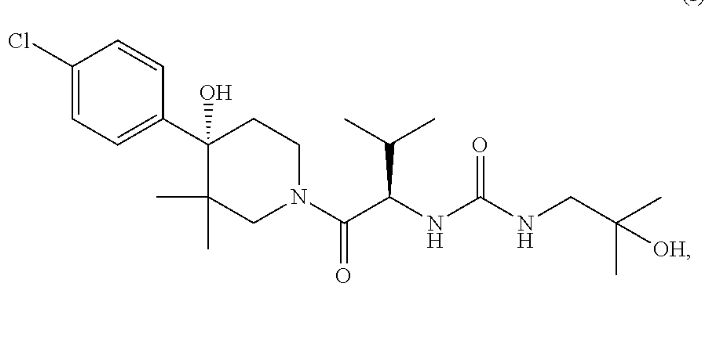
Source: GPS
Key Features of this Related Patent:
- Tablet-based formulation – The reference describes pharmaceutical compositions that can be tabletted, similar to US12178816B2.
- Amorphous solid dispersions – The patent discloses amorphous solid dispersions for improved bioavailability, a core feature of US12178816B2.
- Polymer selection for stabilization – The reference lists polymers such as PVP, HPMC-AS, and HPMC, overlapping with those in US12178816B2.
- Inclusion of pharmaceutical excipients – The patent includes pharmaceutically acceptable excipients, ensuring formulation stability.
Which features of US12178816B2 are disclosed by US20140163071A1?
| Key Feature of Claim 1 | Disclosure Status |
| The pharmaceutical composition is in the form of an immediate-release tablet | Partially Disclosed |
| The composition contains an extrudate comprising 100-200 mg of Compound 1 | Partially Disclosed |
| The extrudate contains at least one polymer from a specified list | Partially Disclosed |
| The extrudate contains at least one glidant | Partially Disclosed |
| The composition contains at least one excipient | Fully Disclosed |
| The weight ratio of Compound 1 to the polymer in the extrudate is from 1:1 to 1:9 | Partially Disclosed |
| The total concentration of Compound 1 in the tablet is from 10% to 35% by weight | Partially Disclosed |
Key Excerpt from US20140163071A1:
“The invention also provides pharmaceutical compositions comprising a pharmaceutically acceptable carrier and a therapeutically effective amount of the amorphous solid dispersion of the present invention… A preferred tabletted embodiment comprises the dispersion in a weight range of 10-75%, preferably 20-60%.”
#3. US6042847A
This patent, filed on November 12, 1997, describes a three-phase pharmaceutical form designed for constant and controlled release of an amorphous active ingredient for single daily application. The formulation includes polyvinylpyrrolidone (PVP) and cellulose ethers, which contribute to the stabilization of the amorphous compound and help control the dissolution rate.
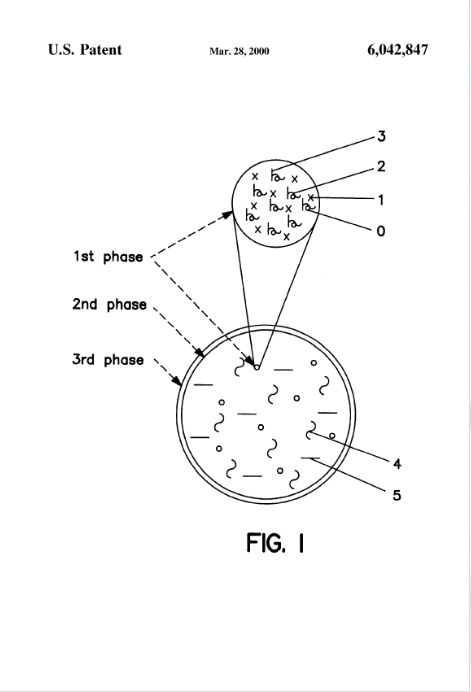
Source: GPS
Key Features of this Related Patent:
- Use of polymers in the pharmaceutical formulation – The reference discloses polyvinylpyrrolidone (PVP) and cellulose ethers, which overlap with the polymer selection in US12178816B2.
- Presence of excipients – The reference describes fillers, binders, and lubricants, aligning with US12178816B2 in terms of formulation components.
- Inclusion of glidants – The formulation includes talc and magnesium stearate, which function as glidants, though their presence in the extrudate is not explicitly confirmed.
Which features of US12178816B2 are disclosed by US6042847A?
| Key feature of claim 1 | Disclosure status |
| The extrudate contains at least one polymer selected from copovidone, povidone, hypromellose phthalate, hypromellose acetate succinate, 2-hydroxypropyl-β-cyclodextrin, hypromellose, polymethacrylates, hydroxypropyl cellulose, and cellulose acetate phthalate | Partially disclosed |
| The extrudate contains at least one glidant | Partially disclosed |
| The composition contains at least one excipient | Fully disclosed |
Key excerpt from US6042847A:
“For providing a stable amorphous form of the active ingredient with a constant dissolution rate, the required weight ratio between the amorphous active ingredient, water-soluble polymer polyvinylpyrrolidone, and cellulose ether ensures prolonged therapeutic action.”
#4. KR20110132116A
This patent, filed on June 1, 2010, describes a solid dispersion comprising raloxifene hydrochloride, along with methods for manufacturing and oral dosage forms incorporating the solid dispersion. The invention focuses on improving solubility and bioavailability of the active ingredient through the use of beta-cyclodextrin (β-cyclodextrin) and hydroxypropyl methylcellulose (HPMC) as dispersing agents.
Key Features of this Related Patent:
- Use of polymers in the pharmaceutical formulation – The reference discloses beta-cyclodextrin (β-cyclodextrin) and hydroxypropyl methylcellulose (HPMC), which overlap with the polymer selection in US12178816B2.
- Presence of excipients – The reference explicitly describes the inclusion of pharmaceutically acceptable excipients, aligning with US12178816B2.
- Total concentration of active ingredient – The reference states that raloxifene hydrochloride makes up approximately 23% of the tablet weight, which falls within the 10%-35% range specified in US12178816B2.
This is how feature mapping from the tool looks like:
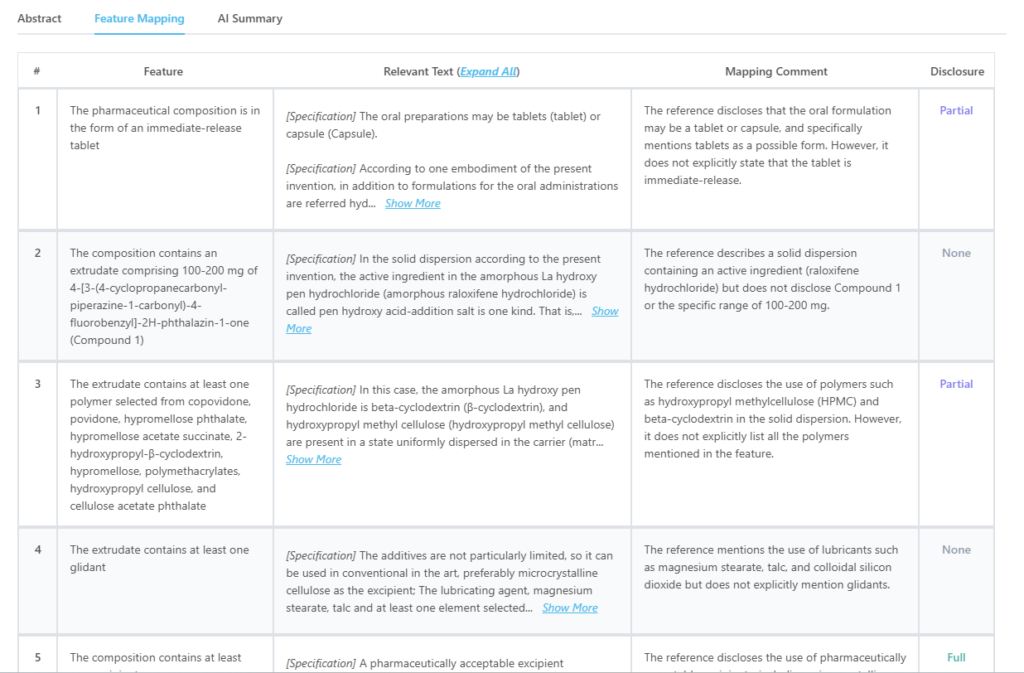
Source: GPS
Which features of US12178816B2 are disclosed by KR20110132116A?
| Key feature of claim 1 | Disclosure status |
| Pharmaceutical composition is in the form of an immediate-release tablet | Partially disclosed |
| The extrudate contains at least one polymer selected from copovidone, povidone, hypromellose phthalate, hypromellose acetate succinate, 2-hydroxypropyl-β-cyclodextrin, hypromellose, polymethacrylates, hydroxypropyl cellulose, and cellulose acetate phthalate | Partially disclosed |
| The composition contains at least one excipient | Fully disclosed |
| The total concentration of Compound 1 in the tablet is from 10% to 35% by weight | Partially disclosed |
Key excerpt from KR20110132116A:
“In the solid dispersion according to the present invention, the active ingredient in the amorphous La hydroxy pen hydrochloride is called pen hydroxy acid-addition salt, which is uniformly dispersed in a matrix comprising beta-cyclodextrin and hydroxypropyl methylcellulose to enhance solubility and bioavailability.”
#5. JP2003531099A
This patent, filed on March 20, 2000, describes a pharmaceutical preparation designed to improve water solubility of poorly water-soluble active pharmaceutical ingredients. The invention involves the formation of solid solutions of active pharmaceutical compounds using stabilizers and excipients to enhance solubility and bioavailability.
Key Features of this Related Patent:
- Use of polymers in the pharmaceutical formulation – The reference describes hydroxypropyl methylcellulose (HPMC) and polyvinylpyrrolidone (PVP) as stabilizers, which overlap with the polymer selection in US12178816B2.
- Presence of excipients – The reference explicitly mentions the use of pharmaceutical excipients such as binders, disintegrants, and stabilizers, aligning with US12178816B2.
- Total concentration of active ingredient – The reference states that itraconazole concentrations range from 20% to 35%, which partially overlaps with the 10%-35% range specified in US12178816B2.
Which features of US12178816B2 are disclosed by JP2003531099A?
| Key feature of claim 1 | Disclosure status |
| Pharmaceutical composition is in the form of an immediate-release tablet | Partially disclosed |
| The extrudate contains at least one polymer selected from copovidone, povidone, hypromellose phthalate, hypromellose acetate succinate, 2-hydroxypropyl-β-cyclodextrin, hypromellose, polymethacrylates, hydroxypropyl cellulose, and cellulose acetate phthalate | Partially disclosed |
| The composition contains at least one excipient | Fully disclosed |
| The total concentration of Compound 1 in the tablet is from 10% to 35% by weight | Partially disclosed |
Key excerpt from JP2003531099A:
“According to the present invention, improved solubility and bioavailability of slightly water-soluble crystalline pharmaceutical activators such as itraconazole are achieved by forming solid solutions using stabilizers such as hydroxypropyl methylcellulose and polyvinylpyrrolidone.”
Feature Comparison Table
| Key feature of claim 1 | RU2440119C2 | US20140163071A1 | US6042847A | KR20110132116A | JP2003531099A |
| Pharmaceutical composition is in the form of an immediate-release tablet | Fully disclosed | Partially disclosed | Not disclosed | Partially disclosed | Partially disclosed |
| The composition contains an extrudate comprising 100-200 mg of 4-[3-(4-cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one (Compound 1) | Partially disclosed | Partially disclosed | Not disclosed | Not disclosed | Not disclosed |
| The extrudate contains at least one polymer selected from copovidone, povidone, hypromellose phthalate, hypromellose acetate succinate, 2-hydroxypropyl-β-cyclodextrin, hypromellose, polymethacrylates, hydroxypropyl cellulose, and cellulose acetate phthalate | Fully disclosed | Partially disclosed | Partially disclosed | Partially disclosed | Partially disclosed |
| The extrudate contains at least one glidant | Partially disclosed | Partially disclosed | Partially disclosed | Not disclosed | Not disclosed |
| The composition contains at least one excipient | Fully disclosed | Fully disclosed | Fully disclosed | Fully disclosed | Fully disclosed |
| The weight ratio of Compound 1 to the polymer in the extrudate is from 1:1 to 1:9 | Partially disclosed | Partially disclosed | Not disclosed | Not disclosed | Not disclosed |
| The total concentration of Compound 1 in the tablet is from 10% to 35% by weight | Partially disclosed | Partially disclosed | Not disclosed | Partially disclosed | Partially disclosed |
How to Identify Related Patents Using Global Patent Search
Evaluating a patent’s validity requires thorough research into related patents. The Global Patent Search (GPS) tool simplifies this process by offering:
- Patent number and description search – Quickly locate relevant patents by entering specific terms or numbers.
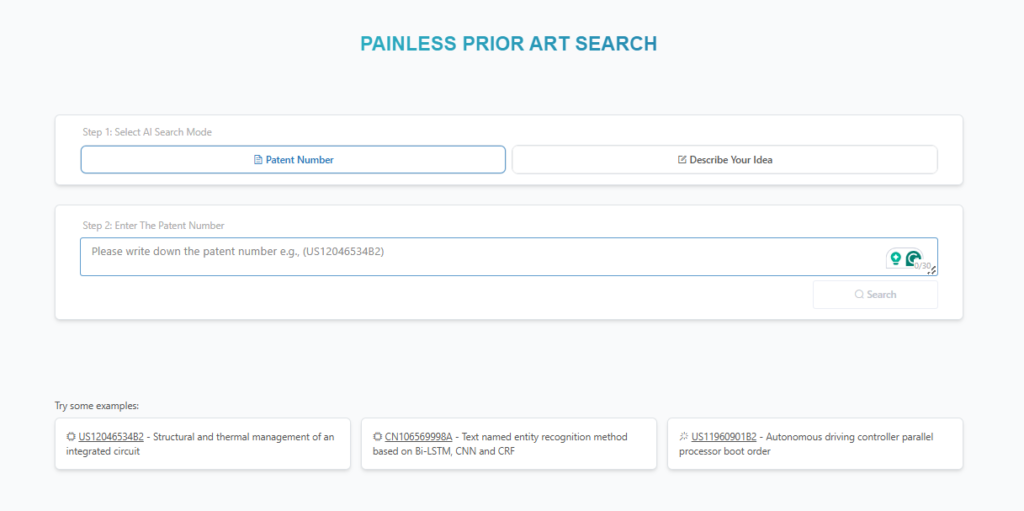
Source: GPS
- Feature mapping analysis – Compare key features of a patent against existing patents to determine overlaps.
- Curated results review – Explore a comprehensive list of related patents that could impact the subject patent.
- Detailed mapping reports – Gain insights into how prior patents correspond to the claims of the subject patent.
- Data-driven decision-making – Strengthen legal strategies with precise and well-documented patent comparisons.
With Global Patent Search, professionals can efficiently uncover related patents that may influence patent litigation and validity assessments.
Take the Guesswork Out of Patent Research with Global Patent Search
Navigating patent disputes requires precision. Finding related patents shouldn’t be a challenge. Global Patent Search (GPS) empowers you with:
- Instant results – Say goodbye to endless manual searches.
- Detailed feature mapping – Pinpoint key similarities with unmatched accuracy.
- Comprehensive insights – Strengthen your case with verified data.
Don’t let critical related patents slip through the cracks. Start your search with Global Patent Search today!
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search (GPS) tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.