Could AstraZeneca Pharmaceuticals’ patent US12144810B1 be facing significant challenges? This patent is currently at the center of extensive litigation, with AstraZeneca pursuing legal action against multiple companies, including Natco Pharma Limited, Sandoz Inc., Cipla Limited, and Zydus Pharmaceuticals (USA) Inc. The disputes involve six plaintiffs, two defendants, two accused products, and five patents-in-suit, underscoring the high stakes of this legal battle.
US12144810B1 pertains to a pharmaceutical formulation designed to enhance drug stability and efficacy. While the patent’s technical details are intricate, its core innovation lies in improving the delivery and performance of specific medications, potentially offering better therapeutic outcomes for patients.
Understanding related patents is crucial in this context, as they can significantly impact the validity and enforcement of a patent under litigation. The Global Patent Search (GPS) tool is instrumental in this analysis, enabling users to identify existing patents that may affect the patent in question.
By leveraging GPS, stakeholders can navigate the complex patent landscape more effectively, ensuring informed decisions in legal strategies.In the following sections, we will delve deeper into the specifics of patent US12144810B1, explore potentially related patents, and discuss how tools like GPS can aid in comprehensive patent analysis.
Understanding Patent US12144810B1
Patent US12144810B1 pertains to a pharmaceutical formulation designed to enhance the bioavailability and stability of 4-[3-(4-cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluoro-benzyl]-2H-phthalazin-1-one (referred to as Compound 1). This compound is a poly(ADP-ribose) polymerase (PARP) inhibitor, used primarily for treating cancers, particularly those associated with BRCA1/BRCA2 mutations.
The invention utilizes a solid dispersion of Compound 1 with a matrix polymer that exhibits low hygroscopicity and high softening temperature, such as copovidone, to overcome limitations in conventional formulations, including low bioavailability and high dosage requirements.
Its four key features are:
#1. Solid dispersion with a matrix polymer: Compound 1 is formulated with a polymeric carrier (e.g., copovidone) to improve solubility and stability.
#2. Improved bioavailability: The solid dispersion significantly enhances absorption compared to immediate-release (IR) tablets or lipid-based formulations.
#3. Optimized drug loading: Allows higher drug concentrations (up to 60% loading) without compromising stability or effectiveness.
#4. Manufacturing via Hot Melt Extrusion (HME): Uses a solvent-free process to create amorphous solid dispersions, reducing the likelihood of crystallization and degradation.
Patent US12144810B1 is significant in pharmaceutical litigation due to its solid dispersion formulation, which enhances drug performance. Legal challenges may question its novelty, non-obviousness, and enablement, particularly whether similar copovidone-based dispersions were previously disclosed. The patent’s bioavailability improvements and claim scope will be key to determining its validity.
Related Patent Reference for US12144810B1
#1. RU2489149C2
This patent, filed on June 5, 2008, describes a solid dispersion formulation aimed at stabilizing the amorphous form of Imatinib mesylate. It utilizes cellulose derivatives, polyvinylpyrrolidone (PVP), and polyethylene glycols (PEG) as excipients to prevent crystallization and enhance bioavailability.
Key Features of this Related Patent:
- Solid dispersion formulation – The reference discloses solid dispersions of amorphous Imatinib mesylate with excipients to enhance drug stability, similar to US12144810B1.
- Use of matrix polymers – It mentions copovidone, hypromellose phthalate, hypromellose acetate succinate, hydroxypropyl cellulose, and cellulose acetate phthalate, overlapping with the polymers listed in US12144810B1.
- Drug loading percentage – The solid dispersion contains 10% to 50% by weight of the active pharmaceutical ingredient, aligning with US12144810B1.
- Polymer-to-drug ratio – The reference specifies weight ratios of 10:90 to 50:50, which partially overlaps with the 1:1 to 1:9 ratio claimed in US12144810B1.
Which features of US12144810B1 are disclosed by RU2489149C2?
| Key Feature of US12144810B1 | Disclosure Status |
| The composition is an immediate-release pharmaceutical tablet | Partially Disclosed |
| The composition contains a solid dispersion of Compound 1 | Not Disclosed |
| The amount of Compound 1 is 100 mg to 200 mg | Not Disclosed |
| The solid dispersion includes at least one polymer from the claimed list | Fully Disclosed |
| The weight ratio of Compound 1 to polymer is 1:1 to 1:9 | Partially Disclosed |
| The total concentration of Compound 1 in the tablet is 10% to 50% by weight | Fully Disclosed |
| The tablet hardness is greater than or equal to 25 N | Not Disclosed |
Key Excerpt from RU2489149C2:
“Solid dispersions of the present invention comprise amorphous Imatinib mesylate and at least one further excipient selected from cellulose derivatives, polyvinylpyrrolidone, polyethyleneglycols of various molecular weights, polymethacrylates, and hypromellose derivatives to enhance stability and prevent crystallization.”
#2. RU2662819C2
This patent, filed on December 12, 2013, describes a solid dispersion formulation containing a tetrazole derivative (HM30181A) as an active ingredient. It aims to improve solubility and stability by incorporating water-soluble polymers such as hypromellose, hydroxypropyl cellulose, and polyvinylpyrrolidone (PVP).
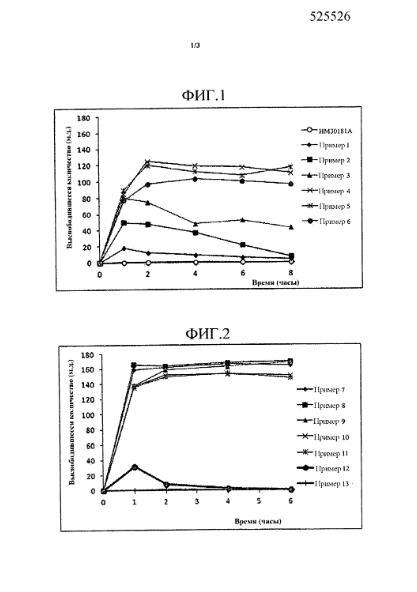
Source: GPS
Key Features of this Related Patent:
- Solid dispersion formulation – The reference discloses solid dispersions containing HM30181A, a tetrazole derivative, to improve drug stability and solubility, similar to US12144810B1.
- Use of matrix polymers – It mentions hypromellose, hydroxypropyl cellulose, and polyvinylpyrrolidone, overlapping with the polymers listed in US12144810B1.
- Drug loading percentage – The tablet formulation contains 10% to 50% by weight of the active ingredient, aligning with US12144810B1.
- Polymer-to-drug ratio – The reference specifies a weight ratio of 1:0.1 to 1:4, which partially overlaps with the 1:1 to 1:9 ratio claimed in US12144810B1.
Which features of US12144810B1 are disclosed by RU2662819C2?
| Key Feature of US12144810B1 | Disclosure Status |
| The composition is an immediate-release pharmaceutical tablet | Partially Disclosed |
| The composition contains a solid dispersion of Compound 1 | Partially Disclosed |
| The solid dispersion includes at least one polymer from the claimed list | Fully Disclosed |
| The weight ratio of Compound 1 to polymer is 1:1 to 1:9 | Partially Disclosed |
| The total concentration of Compound 1 in the tablet is 10% to 50% by weight | Fully Disclosed |
Key Excerpt from RU2662819C2:
“The present invention relates to an amorphous solid dispersion comprising a tetrazole derivative or a pharmaceutically acceptable salt thereof as an active ingredient, combined with a water-soluble polymer such as hypromellose, hydroxypropyl cellulose, or polyvinylpyrrolidone to enhance solubility and stability.”
#3. KR100712146B1
This patent, filed on June 14, 2005, describes a sustained-release pharmaceutical composition containing isradipine formulated with water-soluble polymers to enhance solubility and dissolution rate.
Key Features of this Related Patent:
- Solid dispersion formulation – The reference discloses solid dispersions of isradipine for enhanced dissolution, similar to the formulation approach in US12144810B1.
- Use of matrix polymers – It includes hydroxypropyl cellulose, hydroxypropyl methylcellulose, and povidone, which overlap with the polymers listed in US12144810B1.
- Polymer-to-drug ratio – The reference specifies a weight ratio of 1:0.1 to 1:10, which partially overlaps with the 1:1 to 1:9 ratio claimed in US12144810B1.
- Tablet hardness – The tablet hardness is in the range of 98 to 137 N, which exceeds the minimum 25 N threshold in US12144810B1.
Which features of US12144810B1 are disclosed by KR100712146B1?
| Key Feature of US12144810B1 | Disclosure Status |
| The solid dispersion includes at least one polymer from the claimed list | Partially Disclosed |
| The weight ratio of Compound 1 to polymer is 1:1 to 1:9 | Partially Disclosed |
| The tablet hardness is greater than or equal to 25 N | Partially Disclosed |
Key Excerpt from KR100712146B1:
“The present invention relates to a sustained-release preparation containing isradipine, formulated with water-soluble polymers such as hydroxypropyl cellulose, hydroxypropyl methylcellulose, and povidone to enhance solubility and control drug release over an extended period.”
#4. EP2105130A1
This patent, filed on March 25, 2008, describes a pharmaceutical formulation containing an amorphous solid dispersion of an active ingredient embedded in a polymer matrix. The reference aligns with US12144810B1, particularly in its use of copovidone and other matrix polymers, as well as its approach to stabilizing drug dispersions for improved bioavailability.
Key Features of this Related Patent:
- Solid dispersion formulation – The reference discloses a solid dispersion where the active ingredient is embedded in a polymer matrix, similar to US12144810B1.
- Use of matrix polymers – It includes copovidone, povidone, hypromellose phthalate, hypromellose acetate succinate, hydroxypropyl cellulose, and cellulose acetate phthalate, all of which are claimed in US12144810B1.
- Polymer-to-drug ratio – The reference suggests a weight ratio of active ingredient to polymer that may overlap with the 1:1 to 1:9 range claimed in US12144810B1.
- Active ingredient concentration – The solid dispersion contains 45-55% active ingredient, which partially aligns with the 10% to 50% drug concentration in US12144810B1.
This is how feature mapping from the tool looks like:
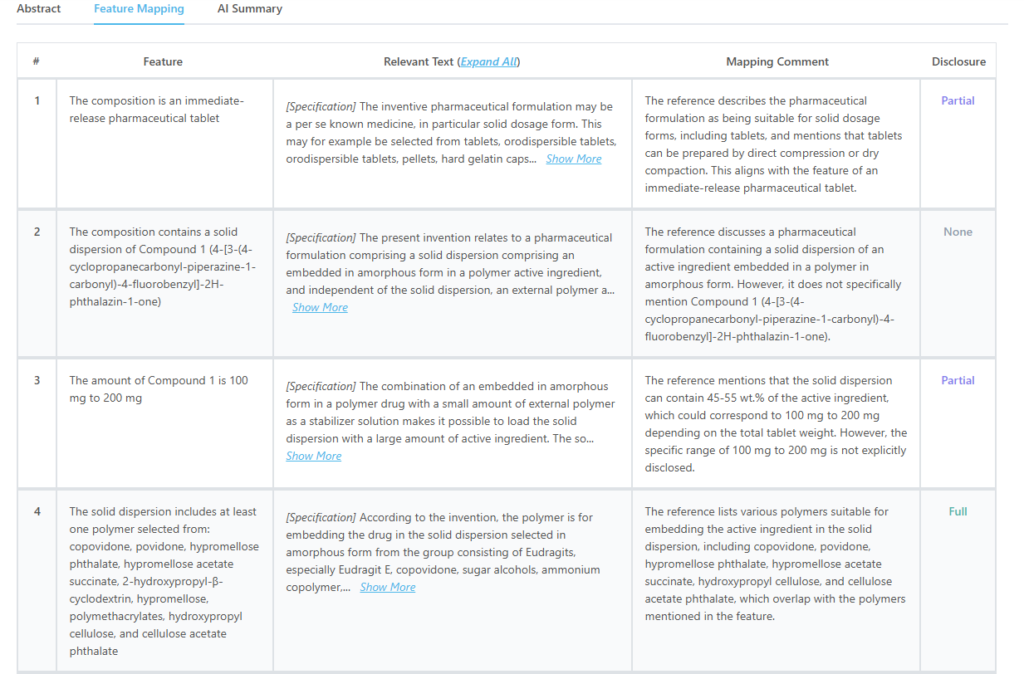
Source: GPS
Which features of US12144810B1 are disclosed by EP2105130A1?
| Key Feature of US12144810B1 | Disclosure Status |
| The composition is an immediate-release pharmaceutical tablet | Partially Disclosed |
| The amount of Compound 1 is 100 mg to 200 mg | Partially Disclosed |
| The solid dispersion includes at least one polymer from the claimed list | Fully Disclosed |
| The weight ratio of Compound 1 to polymer is 1:1 to 1:9 | Partially Disclosed |
| The total concentration of Compound 1 in the tablet is 10% to 50% by weight | Partially Disclosed |
Key Excerpt from EP2105130A1:
“The present invention relates to a pharmaceutical formulation comprising a solid dispersion where the active ingredient is embedded in a polymer in amorphous form, including polymers such as copovidone, povidone, hypromellose phthalate, and cellulose acetate phthalate to improve stability and bioavailability.”
#5. DE102005047561A1
This patent, filed on October 4, 2005, describes a solid, orally administrable pharmaceutical dosage form with immediate release, particularly focusing on fast-release active substances. The reference aligns with US12144810B1 in its immediate-release formulation and use of matrix polymers to stabilize amorphous drug dispersions.
Key Features of this Related Patent:
- Immediate-release tablet formulation – The reference explicitly describes solid, orally administrable pharmaceutical dosage forms with fast-release active substances, matching US12144810B1.
- Use of matrix polymers – The patent mentions hydroxypropyl cellulose, polyvinylpyrrolidone (PVP), polyethylene glycol, and polymethacrylates, partially overlapping with the polymers listed in US12144810B1.
- Tablet hardness – The reference states that the tablet hardness is approximately 40 N, exceeding the 25 N threshold claimed in US12144810B1.
- Solid dispersion for drug stabilization – The active ingredient is in amorphous form within a polymer matrix, similar to the solid dispersion approach of US12144810B1.
Which features of US12144810B1 are disclosed by DE102005047561A1?
| Key Feature of US12144810B1 | Disclosure Status |
| The composition is an immediate-release pharmaceutical tablet | Fully Disclosed |
| The solid dispersion includes at least one polymer from the claimed list | Partially Disclosed |
| The tablet hardness is greater than or equal to 25 N | Fully Disclosed |
Key Excerpt from DE102005047561A1:
“The present invention relates to solid, orally administrable pharmaceutical dosage forms with immediate release, wherein the active ingredient is in amorphous form and stabilized within a polymer matrix such as hydroxypropyl cellulose or polyvinylpyrrolidone to improve bioavailability.”
Feature Comparison Table
| Key Feature of US12144810B1 | RU2489149C2 | RU2662819C2 | KR100712146B1 | EP2105130A1 | DE102005047561A1 |
| The composition is an immediate-release pharmaceutical tablet | Partially Disclosed | Partially Disclosed | Not Disclosed | Partially Disclosed | Fully Disclosed |
| The composition contains a solid dispersion of Compound 1 | Not Disclosed | Partially Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| The amount of Compound 1 is 100 mg to 200 mg | Not Disclosed | Not Disclosed | Not Disclosed | Partially Disclosed | Not Disclosed |
| The solid dispersion includes at least one polymer from the claimed list | Fully Disclosed | Fully Disclosed | Partially Disclosed | Fully Disclosed | Partially Disclosed |
| The weight ratio of Compound 1 to polymer is 1:1 to 1:9 | Partially Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed | Not Disclosed |
| The total concentration of Compound 1 in the tablet is 10% to 50% by weight | Fully Disclosed | Fully Disclosed | Fully Disclosed | Partially Disclosed | Not Disclosed |
| The tablet hardness is greater than or equal to 25 N | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Fully Disclosed |
How to Find Related Patents Using Global Patent Search?
Identifying related patents is essential when evaluating the validity of a patent like US12144810B1. The Global Patent Search (GPS) tool streamlines this process by providing comprehensive feature-based comparisons against existing patents.
Key steps to find Related Patents:
- Search by patent number or description: Instantly retrieve related patents by entering patent number (eg.US12144810B1) or relevant technical terms.
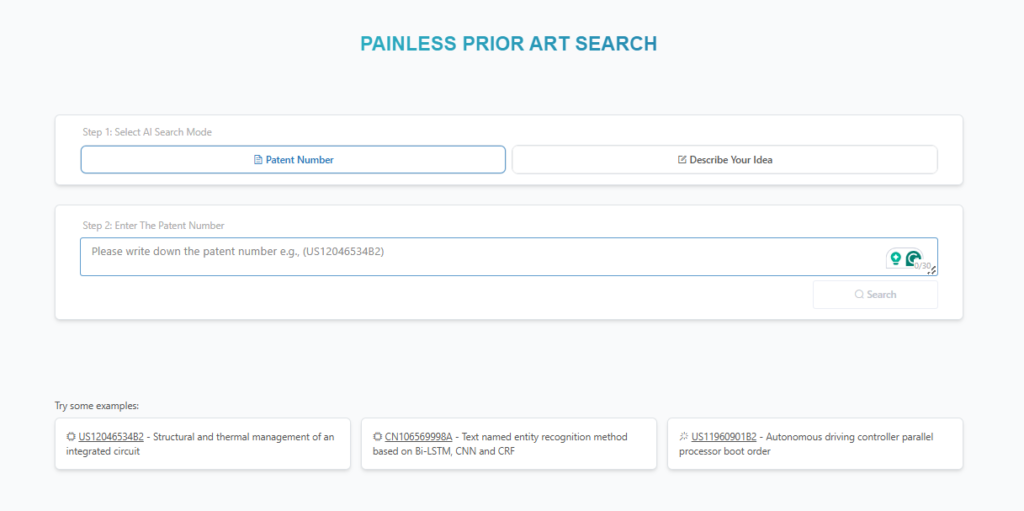
Source: GPS
- Leverage feature mapping: GPS compares key features of the subject patent against a vast patent database to highlight similar disclosures.
- Review matching results: A curated list of potentially related patents is generated, ranking them based on feature similarity and relevance.
- Analyze detailed reports: Each related patent includes a feature mapping table, showing whether it fully, partially, or does not disclose the subject patent’s claims.
- Make data-driven decisions: Use precise data to assess novelty, non-obviousness, and prior disclosures, strengthening patent litigation strategies.
Using Global Patent Search, professionals can efficiently identify, compare, and analyze related patents, ensuring well-informed legal and R&D decisions.
Streamline your Related Patent Research Today
Navigating patent disputes requires precise and reliable data. The Global Patent Search tool helps you:
Identify relevant patents instantly – No more time-consuming manual searches.
Leverage accurate feature mapping – Compare key elements with precision.
Make data-driven decisions – Strengthen your case with verified insights.
Don’t leave your patent research to chance. Start using Global Patent Search now!
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search (GPS) tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.