Did you know that the patent US10383834B2 is currently at the center of a legal battle? US10383834B2, owned by Mallinckrodt Pharmaceuticals Ireland Limited, is currently in litigation against Baxter Healthcare Corporation.
This patent covers a reduced-dose intravenous acetaminophen formulation designed to provide effective pain and fever relief while minimizing the risk of hepatotoxicity.
Understanding the validity of this patent is critical, especially in light of related patent that may disclose similar formulations or treatment methods. If earlier patents or publications demonstrate the same concepts, the patent’s enforceability could be at risk.
This article will analyze potential related patent references that might challenge the uniqueness of US10383834B2, using the Global Patent Search (GPS) tool to compare key features.
Now, let us break down the patent details before diving into the related patent analysis.
Understanding Patent US10383834B2
Patent US10383834B2, titled “Reduced Dose Intravenous Acetaminophen”, describes pharmaceutical compositions and methods for intravenous administration of acetaminophen at a single dose level of less than 1000 mg. The goal is to provide effective pain and fever relief while allowing for more frequent administration without exceeding the 4000 mg daily limit.
Key features of the invention are:
#1. Reduced-dose acetaminophen formulation – The IV formulation contains about 650 mg of acetaminophen, designed for patients weighing at least 50 kg.
#2. Frequent administration protocol – The formulation is administered every four hours, with a maximum daily dose of less than 4000 mg.
#3. IV administration format – The composition is provided as either a sterile solution or a lyophilized powder that is reconstituted before use.
#4. Targeted pain and fever relief – The method is specifically intended for treating acute pain, postoperative pain, and fever.
Mallinckrodt Pharmaceuticals has enforced US10383834B2 against Baxter Healthcare Corporation. They argue that Baxter’s products infringe upon this reduced-dose IV acetaminophen method. A key factor in this case is whether related patent discloses similar formulations or dosing strategies, which could impact the patent’s validity.
Potential Related Patent References for US10383834B2
#1. CA2705733A1
This patent, filed on Nov. 13, 2008, discloses a reduced-dose intravenous acetaminophen formulation for treating pain and fever. The invention allows more frequent dosing by administering less than 1000 mg per dose, with a total daily limit of less than 4000 mg. It describes formulations in both sterile solution and lyophilized powder forms.
Why this qualifies as Potential Related Patent?
- Reduced-dose IV acetaminophen – The reference describes IV acetaminophen formulations with doses between 600 mg and 700 mg, aligning with the claimed 650 mg dose in US10383834B2.
- Treatment of pain and fever – The patent explicitly mentions pain relief, including acute pain, postoperative pain, and fever reduction, which corresponds to US10383834B2.
- Intravenous administration – The formulation is provided as a sterile IV solution or lyophilized powder for reconstitution, matching the method in US10383834B2.
- Four-hour dosing intervals – The reference states that administration can occur every 3 to 5 hours, which includes the four-hour interval claimed in US10383834B2.
- Maximum daily dose under 4000 mg – The prior art specifies that total acetaminophen administration remains below 4000 mg per day, consistent with US10383834B2.
Which Features of US10383834B2 are disclosed in CA2705733A1?
| Key Feature of Claim 1 | Disclosure Status |
| The subject is a human weighing at least 50 kg | Fully Disclosed |
| The treatment is for pain | Fully Disclosed |
| The formulation contains about 650 mg of acetaminophen | Fully Disclosed |
| The formulation is administered intravenously | Fully Disclosed |
| The administration is repeated every four hours | Fully Disclosed |
| The maximum daily dose is less than 4000 mg of acetaminophen per day | Fully Disclosed |
Key Excerpt from CA2705733A1:
“In some embodiments, the administration is repeated at least once with an interval of about 3 to about 5 hours. In some embodiments, the administration is repeated at least six times in a period of twenty-four hours, delivering less than 4000 mg of acetaminophen.”
#2. WO2008079818A2
This patent, filed on Dec. 18, 2007, discloses intravenous analgesic formulations, including aspirin and acetaminophen, for treating pain and other medical conditions. The invention focuses on aqueous formulations designed for IV administration to ensure stability, bioactivity, and gastrointestinal safety.
Why this qualifies as Potential Related Patent?
- Intravenous analgesic formulations – The reference describes IV formulations containing various analgesics, including acetaminophen, making it relevant to US10383834B2.
- Treatment of pain – The patent explicitly mentions pain relief, aligning with the purpose of US10383834B2.
- IV administration method – The invention ensures stability and bioactivity in IV-administered analgesic formulations, consistent with US10383834B2.
Which Features of US10383834B2 are disclosed in WO2008079818A2?
| Key Feature of Claim 1 | Disclosure Status |
| The treatment is for pain | Fully Disclosed |
| The formulation is administered intravenously | Fully Disclosed |
Key Excerpt from WO2008079818A2:
“The present invention provides formulations of analgesics suitable for intravenous administration, methods of formulating analgesics for intravenous administration, as well as methods of using the formulations of analgesics suitable for intravenous administration to treat or prevent various diseases or medical conditions.”
#3. US20020032215A1
This patent, filed on May 17, 2001, discloses a combination therapy for pain treatment involving acetaminophen and azapirone compounds. The invention describes various routes of administration, including parenteral, rectal, and oral, with the goal of achieving enhanced opioid-like analgesia while minimizing toxicity.
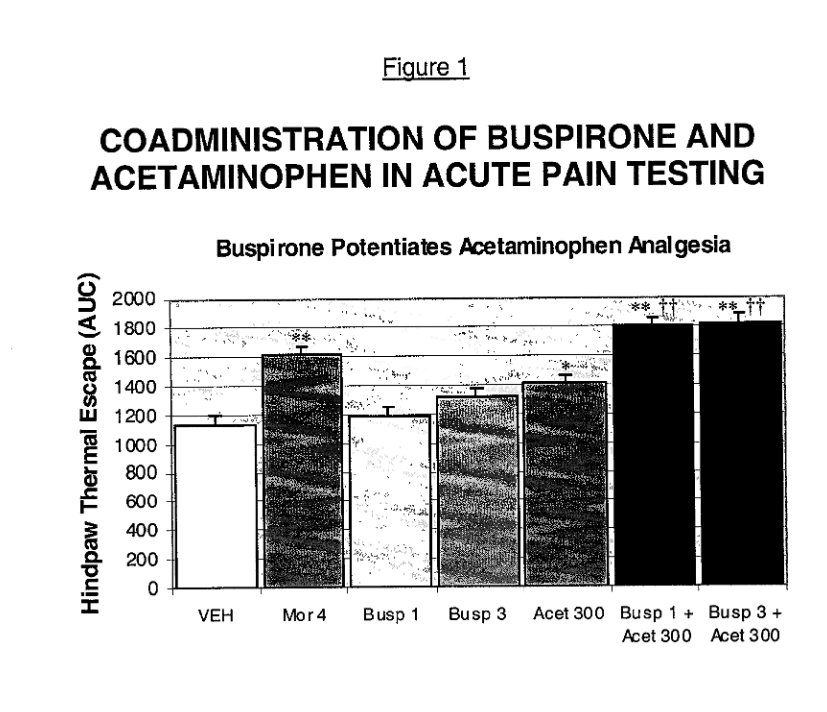
Source – US20020032215A1
Why this qualifies as Potential Related Patent?
- Pain treatment using acetaminophen – The reference explicitly states that acetaminophen is used for pain relief, aligning with US10383834B2.
- Dosage range includes 650 mg – The reference mentions that acetaminophen is typically administered in doses between 600 mg and 1300 mg per dose, which includes the 650 mg dose claimed in US10383834B2.
- Daily dose aligns with subject patent – The reference specifies that acetaminophen administration does not exceed 4000 mg per day, consistent with US10383834B2.
Which Features of US10383834B2 are disclosed in US20020032215A1?
| Key Feature of Claim 1 | Disclosure Status |
| The treatment is for pain | Fully Disclosed |
| The formulation contains about 650 mg of acetaminophen | Partially Disclosed |
| The maximum daily dose is less than 4000 mg of acetaminophen per day | Fully Disclosed |
Key Excerpt from US20020032215A1:
“Acetaminophen by itself is generally given in analgesic doses ranging from about 300 to 1300 mg and preferably from about 650 to 1300 mg with a maximum recommended daily dose of about 4000 mg.”
#4. JPH09143074A
This patent, filed on Nov 20, 1995, discloses a prolonged-action cold medicine tablet containing acetaminophen, dihydrocodeine phosphate, and dl-methylephedrine hydrochloride. The formulation is designed for twice-daily oral administration, ensuring sustained release for extended symptom relief.
Why this qualifies as Potential Related Patent?
- Acetaminophen-based formulation – The reference describes a cold medicine formulation containing acetaminophen, which partially overlaps with US10383834B2.
- Pain relief as a secondary function – Although primarily for cold symptoms, the presence of acetaminophen suggests potential pain relief properties.
- Controlled daily dosage – The reference ensures a daily acetaminophen intake below 4000 mg, which aligns with US10383834B2.
Here’s what the mapping from the tool for this particular patent looks like:
Source – GPS
Which Features of US10383834B2 are disclosed in JPH09143074A?
| Key Feature of Claim 1 | Disclosure Status |
| The treatment is for pain | Partially Disclosed |
| The maximum daily dose is less than 4000 mg of acetaminophen per day | Fully Disclosed |
Key Excerpt from JPH09143074A:
“In the long-acting tablets of the present invention, the blending amount of acetaminophen, dihydrocodeine phosphate, and dl-methylephedrine hydrochloride tricomponent is not particularly limited. However, it is preferable that the dose at the time of single administration is an amount that can be expected to have a certain effect by taking twice a day. Specifically, it is preferable to mix 1/2 of the maximum daily dose specified in the over-the-counter drug manufacturing (import) approval standard for cold medicine.”
#5. WO9748390A1
This patent, filed on June 20, 1997, discloses analgesic compositions containing acetaminophen and meclizine for the treatment of mild to moderately severe pain. The formulations include tablets, elixirs, and other oral dosage forms, with recommended administration intervals of 4 to 6 hours and a daily acetaminophen intake limit of 4000 mg.
Why this qualifies as Potential Related Patent?
- Pain relief using acetaminophen – The reference explicitly states that acetaminophen is used for analgesia, aligning with US10383834B2.
- Dosing frequency partially matches – The reference specifies that acetaminophen formulations can be administered every 4 to 6 hours, which partially aligns with the four-hour dosing interval in US10383834B2.
- Daily dose limit is consistent – The reference states that total acetaminophen administration does not exceed 4000 mg per day, consistent with US10383834B2.
Which Features of US10383834B2 are disclosed in WO9748390A1?
| Key Feature of Claim 1 | Disclosure Status |
| The subject is a human weighing at least 50 kg | Partially Disclosed |
| The treatment is for pain | Fully Disclosed |
| The administration is repeated every four hours | Partially Disclosed |
| The maximum daily dose is less than 4000 mg of acetaminophen per day | Fully Disclosed |
Key Excerpt from WO9748390A1:
“The following are more specific examples of embodiments of the present invention: TABLET 1 – 500 mg APAP and 25 mg meclizine in a 625 mg tablet, 1 or 2 tablets to be administered to an average adult every 4 to 6 hours, not to exceed 8 tablets daily.”
Feature Comparison Table
| Key Feature of Claim 1 | CA2705733A1 | WO2008079818A2 | US20020032215A1 | JPH09143074A | WO9748390A1 |
| The subject is a human weighing at least 50 kg | Fully Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Partially Disclosed |
| The treatment is for pain | Fully Disclosed | Fully Disclosed | Fully Disclosed | Partially Disclosed | Fully Disclosed |
| The formulation contains about 650 mg of acetaminophen | Fully Disclosed | Not Disclosed | Partially Disclosed | Not Disclosed | Not Disclosed |
| The formulation is administered intravenously | Fully Disclosed | Fully Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| The administration is repeated every four hours | Fully Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Partially Disclosed |
| The maximum daily dose is less than 4000 mg of acetaminophen per day | Fully Disclosed | Not Disclosed | Fully Disclosed | Fully Disclosed | Fully Disclosed |
How to Find Related Patents Using Global Patent Search
Patent litigation is a high-stakes battle, and related patent is often the decisive factor that can make or break a case. But finding the right related patent is not about endless searches or sifting through mountains of irrelevant patents. It is about precision, speed, and strategic analysis.
That is where the Global Patent Search (GPS) tool comes in. The benefits are:
Instant access to relevant patents: Forget the days of manually digging through archives. Simply enter the patent number (US10383834B2) or targeted keywords like “intravenous acetaminophen” and instantly retrieve a curated list of potential related patent.
Source: GPS
Feature-to-feature mapping: Not all related patent is created equal. The GPS tool breaks down each reference, mapping core claims to prior publications so you can immediately see where features align and where they don’t.
Deep-dive patent reports: More than just a summary, GPS delivers detailed insights. With full-text excerpts, it helps you build a solid related patent argument backed by data, not guesswork.
Data-driven litigation strategy: Armed with objective comparisons and disclosure status reports, you can confidently challenge a patent’s novelty. Whether it’s an IPR filing or a district court invalidity case, GPS helps uncover the most impactful related patent before the other side does.
The right related patent can shift the entire trajectory of a patent dispute. Make sure you have the best tools like Global Patent Search at your disposal.
Find the Related Patent That Can Strengthen Your Case
Navigating patent litigation requires more than just surface-level research. It demands precise, well-documented related patent. The Global Patent Search (GPS) tool simplifies this process by delivering:
#1. Comprehensive search results – Instantly uncover relevant patents and publications without the hassle of manual digging.
#2. Detailed feature comparisons – Identify overlaps and discrepancies between the disputed patent and related patent with structured claim mapping.
#3. Legal insights – Access critical patent details, excerpts, and disclosure analysis to build a stronger case.
Whether you are challenging a patent’s validity in IPR, district court litigation, or strategic analysis, having the right related patent could make all the difference. Start your search with Global Patent Search today.
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search (GPS) tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.