The patent US10993952B2, owned by Ingenus Pharmaceuticals LLC, is currently under litigation in the case Ingenus Pharmaceuticals, LLC v. Hetero USA, Inc. et al. This patent covers a stable, ready-to-use liquid formulation of cyclophosphamide, an important chemotherapy drug. Unlike conventional lyophilized formulations that require reconstitution before use, this invention focuses on a pre-dissolved liquid composition with a specific solvent system to enhance stability and ease of administration.
Related patent is critical in determining whether this patent can withstand legal scrutiny. If earlier publications or patents disclose similar formulations, the validity of US10993952B2 could be questioned. This is where Global Patent Search (GPS) becomes essential, helping identify potential related patent references that might challenge the novelty of this formulation.
In this article, we’ll break down the patent’s key claims and analyze five potential related patent references that could impact its validity.
Understanding Patent US10993952B2
Patent US10993952B2 covers a stable, ready-to-use liquid formulation of cyclophosphamide for parenteral (injectable) use. Traditional cyclophosphamide formulations are typically lyophilized (freeze-dried) and require reconstitution before administration.
This patent aims to improve convenience and stability by providing a pre-dissolved formulation using a specific combination of solvents and antioxidants.
Source: Google Patents
The key features of this patent include:
#1. Stable liquid formulation – Cyclophosphamide is pre-dissolved in a solvent system to eliminate the need for reconstitution.
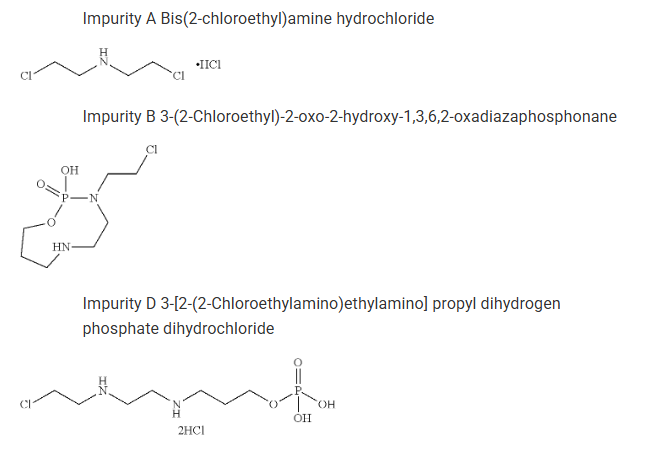
#2. Controlled impurities – The formulation ensures that impurities A, B, and D remain below 0.5%, even under accelerated storage conditions.
#3. Solvent composition – The liquid formulation contains a specific ratio of ethanol (70-75%), polyethylene glycol, and propylene glycol to enhance stability.
#4. Incorporation of antioxidants – Antioxidants such as monothioglycerol are included to prevent degradation and extend shelf life.
If related patent references can demonstrate that similar ready-to-use cyclophosphamide formulations existed before this patent’s filing, the claims could be invalidated.
Potential Related Patent References for US10993952B2
#1. IN735CH2015A
This patent, filed on Feb. 16, 2015, describes stable, ready-to-use liquid formulations of cyclophosphamide for parenteral use. The formulation consists of cyclophosphamide dissolved in a solvent system.
It also includes ethanol, polyethylene glycol, and propylene glycol, designed to improve stability. The reference also provides stability data demonstrating impurity control after storage at 40°C and 75% RH for 7 days.
Why this qualifies as Potential Related Patent?
- Cyclophosphamide concentration (12-23%) – The reference mentions cyclophosphamide in the formulation but does not specify the concentration range of 12-23%.
- Ethanol concentration (70-75%) – Ethanol is listed as a solvent, but no specific concentration range of 70-75% is provided.
- Use of both polyethylene glycol and propylene glycol – Explicitly included in the formulation.
- Polyethylene glycol to propylene glycol ratio (1.0:1.0 to 2.0:1.0) – No specific mass ratio is disclosed.
- Stability against impurities – Discloses impurity levels below 0.5% for Impurities A, B, and D after 7 days of storage under similar conditions.
Which features of US10993952B2 are disclosed by IN735CH2015A?
| Key Feature of Claim 1 | Disclosure Status |
| Cyclophosphamide concentration (12-23%) | Partially Disclosed |
| Ethanol concentration (70-75%) | Partially Disclosed |
| Use of both polyethylene glycol and propylene glycol | Fully Disclosed |
| Stability against Impurity A (bis(2-chloroethyl)amine hydrochloride) | Fully Disclosed |
| Stability against Impurity B (3-(2-chloroethyl)-2-oxo-2-hydroxy-1,3,6,2-oxadiazaphosphonane) | Fully Disclosed |
| Stability against Impurity D (3-[2-(2-chloroethylamino)ethyl amino] propyl dihydrogen phosphate dihydrochloride) | Fully Disclosed |
Key Excerpt from IN735CH2015A:
“The formulations show less than 0.5% each of impurities A, B, and D after being stored at 40°C, 75% RH for 7 days.”
#2. WO2016132270A1
This patent, filed on Feb. 15, 2016, describes stable, ready-to-use liquid formulations of cyclophosphamide for parenteral use. The formulation consists of cyclophosphamide dissolved in a solvent system. It also includes ethanol, polyethylene glycol, and propylene glycol, designed to improve stability. The reference also provides stability data demonstrating impurity control after storage at 40°C and 75% RH for 7 days.
Why this qualifies as Potential Related Patent?
- Stable liquid formulation – Describes ready-to-use liquid cyclophosphamide stored under similar conditions.
- Use of ethanol as a solvent – Discloses ethanol, but does not specify the claimed 70-75% concentration.
- Combination of polyethylene glycol and propylene glycol – Explicitly included in the formulation.
- Polyethylene glycol to propylene glycol ratio – No specific mass ratio is disclosed.
- Impurity control after stability testing – Discloses impurity levels below 0.5% after 7 days of storage under similar conditions.
Which features of US10993952B2 are disclosed by WO2016132270A1?
| Key Feature of Claim 1 | Disclosure Status |
| Cyclophosphamide concentration (12-23%) | Fully Disclosed |
| Ethanol concentration (70-75%) | Partially Disclosed |
| Use of both polyethylene glycol and propylene glycol | Fully Disclosed |
| Polyethylene glycol concentration (3.4-8.8%) | Fully Disclosed |
| Propylene glycol concentration (3.4-4.4%) | Fully Disclosed |
| Stability against Impurity A (bis(2-chloroethyl)amine hydrochloride) | Fully Disclosed |
| Stability against Impurity B (3-(2-chloroethyl)-2-oxo-2-hydroxy-1,3,6,2-oxadiazaphosphonane) | Fully Disclosed |
| Stability against Impurity D (3-[2-(2-chloroethylamino)ethyl amino] propyl dihydrogen phosphate dihydrochloride) | Fully Disclosed |
Key Excerpt from WO2016132270A1:
“The formulations show less than 0.5% each of impurities A, B, and D after being stored at 40°C, 75% RH for 7 days.”
#3. ES2369776T3
This patent, filed on Sept. 28, 2004, describes aqueous concentrates of ifosfamide stabilized with Mesna to prevent decomposition and crystallization. The formulation achieves high-concentration solutions of ifosfamide. It is similar to cyclophosphamide in chemical structure and function and ensures stability during storage.
Why this qualifies as Potential Related Patent?
- Cyclophosphamide concentration (12-23%) – Discloses ifosfamide concentrations ranging from 10% to 50%, including 12%, which overlaps with the claimed range. However, cyclophosphamide is not explicitly mentioned.
- Ethanol concentration (70-75%) – Ethanol is listed as a possible excipient, but no specific concentration is disclosed.
- Use of both polyethylene glycol and propylene glycol – Polyethylene glycol is mentioned, but propylene glycol is not disclosed.
- Polyethylene glycol to propylene glycol ratio (1.0:1.0 to 2.0:1.0) – No ratio is provided.
- Stability against impurities – Discusses chemical stabilization of ifosfamide to prevent decomposition, but does not provide impurity data for bis(2-chloroethyl)amine hydrochloride, 3-(2-chloroethyl)-2-oxo-2-hydroxy-1,3,6,2-oxadiazaphosphonane, or 3-[2-(2-chloroethylamino)ethyl amino] propyl dihydrogen phosphate dihydrochloride.
Here is the mapping from the GPS tool for this particular patent:
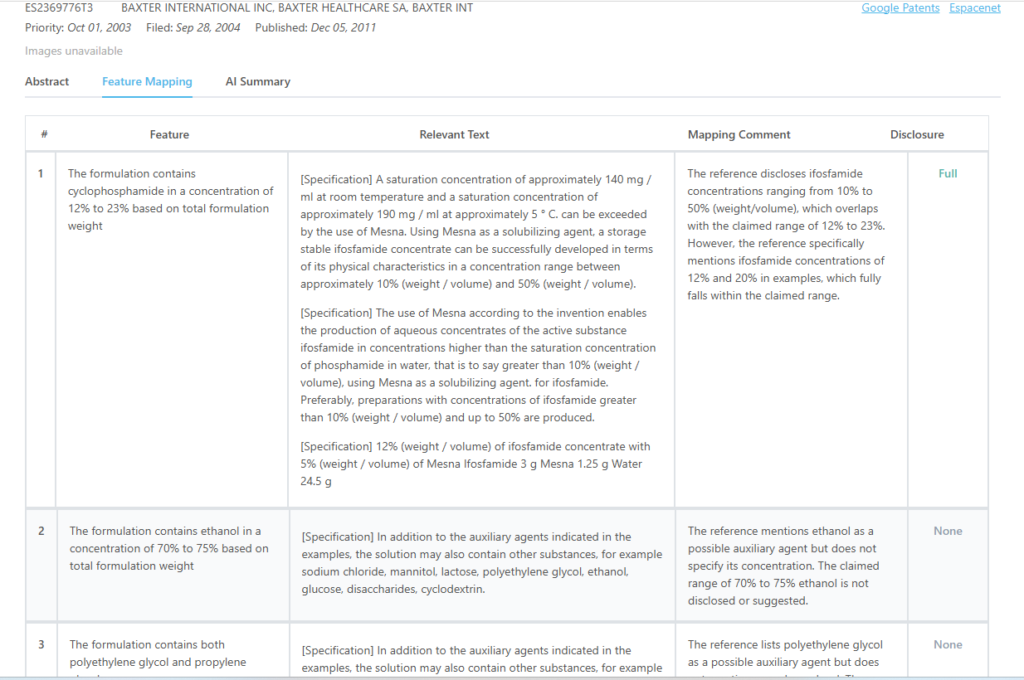
Source – GPS
Which features of US10993952B2 are disclosed by ES2369776T3?
| Key Feature of Claim 1 | Disclosure Status |
| Cyclophosphamide concentration (12-23%) | Partially Disclosed |
Key Excerpt from ES2369776T3:
“A saturation concentration of approximately 140 mg/ml at room temperature and a saturation concentration of approximately 190 mg/ml at approximately 5°C can be exceeded by the use of Mesna. Using Mesna as a solubilizing agent, a storage stable ifosfamide concentrate can be successfully developed.”
#4. CN101862319A
This patent, filed on June 28, 2010, describes a composition for injection containing Docetaxel, focused on improving stability and solubility. The invention primarily relates to Docetaxel formulations and does not mention cyclophosphamide.
Why this qualifies as Potential Related Patent?
- Cyclophosphamide concentration (12-23%) – The reference does not mention cyclophosphamide or its concentration range.
- Ethanol concentration (70-75%) – Ethanol is mentioned as a solvent, but no specific concentration range is provided.
- Use of both polyethylene glycol and propylene glycol – The reference does not mention either polyethylene glycol or propylene glycol.
- Polyethylene glycol to propylene glycol ratio (1.0:1.0 to 2.0:1.0) – No ratio is provided.
- Stability against impurities – The reference does not discuss decomposition products related to cyclophosphamide or impurity levels after storage.
Which features of US10993952B2 are disclosed by CN101862319A?
| Key Feature of Claim 1 | Disclosure Status |
| Ethanol concentration (70-75%) | Partially Disclosed |
Key Excerpt from CN101862319A:
“The invention of the enhanced Daussy device for and matched injection the composition of the preparation process stability, the preparation and matched with the related compositions material contains is lower is Daussy for injection, the quality of homogeneous, the ethanol and drying, wherein transporting and stability of storage process is located.”
#5. US20030055023A1
This patent, filed on March 19, 2002, describes formulations containing etomidate, a poorly water-soluble drug, aimed at improving solubility and stability. The invention includes propylene glycol, polyethylene glycol, ethanol, and other excipients. However, the reference does not mention cyclophosphamide.
Why this qualifies as Potential Related Patent?
- Cyclophosphamide concentration (12-23%) – The reference does not mention cyclophosphamide or its concentration.
- Ethanol concentration (70-75%) – Ethanol is mentioned as a solvent, but no specific concentration range is provided.
- Use of both polyethylene glycol and propylene glycol – Mentions formulations containing either polyethylene glycol or propylene glycol, but does not confirm both are present together in the same formulation.
- Polyethylene glycol to propylene glycol ratio (1.0:1.0 to 2.0:1.0) – No ratio is provided.
- Stability against impurities – The reference does not discuss decomposition products related to cyclophosphamide or impurity levels after storage.
Which features of US10993952B2 are disclosed by US20030055023A1?
| Key Feature of Claim 1 | Disclosure Status |
| Ethanol concentration (70-75%) | Partially Disclosed |
| Use of both polyethylene glycol and propylene glycol | Partially Disclosed |
| Propylene glycol concentration (3.4-4.4%) | Partially Disclosed |
Key Excerpt from US20030055023A1:
“Commercially available formulations of etomidate are clear, colorless solutions containing etomidate, propylene glycol and water. The currently marketed AMIDATE® (Abbott Laboratories, Abbott Park, Ill.), HYPNOMIDATE® (Janssen Pharmaceutica Ltd., South Africa), and Etomidate Injection formulations contain 2 mg/mL of etomidate dissolved in 35% vol. propylene glycol and water.”
Feature Comparison Table
| Feature | IN735CH2015A | WO2016132270A1 | ES2369776T3 | CN101862319A | US20030055023A1 |
| Cyclophosphamide concentration (12-23%) | Partially Disclosed | Fully Disclosed | Partially Disclosed | Not Disclosed | Not Disclosed |
| Ethanol concentration (70-75%) | Partially Disclosed | Partially Disclosed | Not Disclosed | Partially Disclosed | Partially Disclosed |
| Use of both polyethylene glycol and propylene glycol | Fully Disclosed | Fully Disclosed | Not Disclosed | Not Disclosed | Partially Disclosed |
| PEG to propylene glycol mass ratio (1.0:1.0 to 2.0:1.0) | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| Polyethylene glycol concentration (3.4-8.8%) | Not Disclosed | Fully Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| Propylene glycol concentration (3.4-4.4%) | Not Disclosed | Fully Disclosed | Not Disclosed | Not Disclosed | Partially Disclosed |
| Stability against Impurity A (bis(2-chloroethyl)amine hydrochloride) | Fully Disclosed | Fully Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| Stability against Impurity B (3-(2-chloroethyl)-2-oxo-2-hydroxy-1,3,6,2-oxadiazaphosphonane) | Fully Disclosed | Fully Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| Stability against Impurity D (3-[2-(2-chloroethylamino)ethyl amino] propyl dihydrogen phosphate dihydrochloride) | Fully Disclosed | Fully Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
How to Find Related Patent Using Global Patent Search
Related patent plays a crucial role in evaluating a patent’s validity. The Global Patent Search (GPS) tool simplifies this process by offering detailed, data-driven insights to efficiently uncover relevant related patent references. Here’s how it helps:
Search using Patent numbers or description – Enter a patent number (e.g., US10993952B2) or related technical terms to quickly locate relevant patents and published applications.
Source: GPS
Utilize feature mapping – Compare the core technical elements of the patent against existing related patent to determine similarities and distinctions.
Explore matching references – Access a comprehensive list of related patent references, ranked based on their relevance to the patent in question.
Examine detailed analysis – Review how each reference discloses specific features and assess whether they challenge the patent’s novelty or non-obviousness.
Strengthen legal strategies – Leverage fact-based insights to support litigation, evaluate patent strength, and make informed legal decisions.
By using GPS, professionals can conduct thorough, precise related patent searches, ensuring a data-backed approach to patent analysis.
Win Your Patent Case with Data-Backed Related Patent Research
In high-stakes patent litigation, the right related patent can mean the difference between success and failure. Global Patent Search (GPS) provides instant, precise, and comprehensive related patent analysis, giving you the power to challenge patent validity with confidence.
With GPS, you gain:
#1. Immediate access to solid related patent – Eliminate guesswork and find the most relevant references instantly.
#2. Precision feature mapping – Identify key overlaps between the disputed patent and existing related patent with unmatched accuracy.
#3. Data-driven legal advantage – Strengthen your case with high-relevance related patent that can undermine patent claims.
The right related patent can dismantle a patent. Get the evidence you need; start your search with Global Patent Search now.
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search (GPS) tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.