Astellas Pharma Inc. has initiated legal action against both Lupin Limited and Zydus Pharmaceuticals over patent US12097189B1. These lawsuits center around a modified-release pharmaceutical composition designed to optimize drug absorption and therapeutic efficacy.
The invention focuses on precisely controlling the release of a compound with potential applications in treating diabetes and overactive bladder. The innovation aims to eliminate food effects on drug absorption, ensuring consistent therapeutic efficacy.
But does this innovation truly stand on solid ground? Related Patent is the ultimate test. If earlier technologies describe similar sustained-release formulations or methods to reduce food effects, Astellas’ claims could face serious challenges.
Using Global Patent Search (GPS), we have identified five potential related patent references that could threaten the novelty of this patent. Before we dive into them, let us break down the patent’s core features.
Understanding US Patent 12097189B1
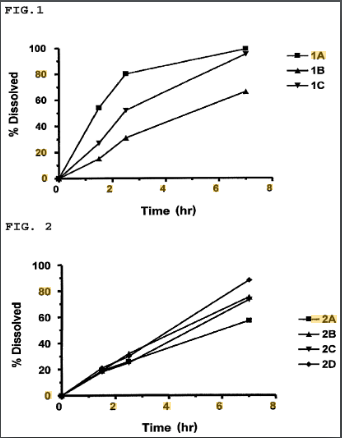
US12097189B1 describes a modified-release pharmaceutical composition designed to control the drug release rate of (R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetic acid anilide.
The primary objective is to minimize the impact of food on drug absorption, ensuring consistent bioavailability and therapeutic efficacy.
Source: US12097189B1
Key features of the patent are:
#1. Controlled-release formulation – The composition regulates the drug’s release rate, optimizing absorption.
#2. Food-effect minimization – The formulation is designed to mitigate variations in drug absorption caused by food intake.
#3. Sustained drug levels – Ensures a steady plasma concentration for improved efficacy.
#4. Pharmaceutical carrier system – Utilizes excipients to achieve the desired release profile.
Astellas Pharma is asserting that both Lupin Limited and Zydus Pharmaceuticals infringes on this patent, claiming their product employs similar release mechanisms. However, if prior patents or scientific disclosures reveal comparable controlled-release formulations, Astellas’ claims may weaken.
Potential Related Patent References for US12097189B1
#1. KR20130106456A
This patent, filed on April 29, 2011, discloses a sustained-release drug formulation. The formulation incorporates carriers to modulate drug absorption and optimize therapeutic efficacy. While the reference focuses on sustained-release technology, it does not explicitly discuss reducing food effects or treating overactive bladder.
Source: GPS
Why this qualifies as Potential Related Patent?
- Sustained-release drug formulation – The reference describes a controlled-release composition designed to prolong drug release and reduce side effects, aligning with the core concept of US12097189B1.
- Oral tablet administration – The reference specifies sustained-release tablets as an oral dosage form, corresponding to the administration method claimed in US12097189B1.
- Use of hydrogel-forming polymers – The reference incorporates hydrogel-forming materials such as HPMC, which matches the formulation approach in US12097189B1.
- Inclusion of excipients for controlled release – The prior art discusses carriers, solubilizers, and polymers designed to control drug release, similar to the composition in US12097189B1.
- Sustained drug release – The reference describes continuous drug release over time, but does not specify a minimum of 4 hours, as required by US12097189B1.
Which features of US12097189B1 are disclosed in KR20130106456A?
| Key Feature of Claim 1 | Disclosure Status |
| The method comprises oral administration of a tablet | Partially Disclosed |
| The formulation is a sustained-release hydrogel-forming formulation | Fully Disclosed |
| The formulation comprises a carrier | Fully Disclosed |
| The formulation provides continuous drug release for at least 4 hours after oral administration | Partially Disclosed |
Key Excerpt from KR20130106456A
“Sustained-release compared to the conventional immediate release (rapid release) formulation (controlled release) preparations may be maintained so as to continuously release the drug for a period of time in the body, an effect that can reduce side effects and improve patient compliance.”
#2. AU6802094A
This patent, filed on May 27, 1994, discloses a drug delivery composition for alpha-adreno receptor blocking agents. It is specifically designed for controlled and sustained drug release. While the invention focuses on alfuzosin hydrochloride, it does not explicitly discuss the treatment of overactive bladder or the reduction of food effects in a quantitative manner.
Why this qualifies as Potential Related Patent?
- Oral administration of a tablet – The reference describes tablets and microcapsules as part of the drug delivery system, aligning with US12097189B1.
- Sustained-release hydrogel-forming formulation – The invention includes controlled-release systems using hydrophilic gel matrices, which corresponds to US12097189B1.
- Use of carriers – The reference describes microcrystalline cellulose (Avicel) and hydroxypropylmethylcellulose (Methocel K100M) as carriers, which are commonly used in sustained-release drug formulations.
- Sustained drug release for at least 4 hours – The patent specifies that the formulation allows prolonged release over several hours, meeting the sustained-release criterion of US12097189B1.
- Comparison of bioavailability between formulations – The reference discusses the bioavailability of alfuzosin hydrochloride and its absorption characteristics but does not explicitly disclose a quantified reduction in food effect.
Which features of US12097189B1 are disclosed in AU6802094A?
| Key Feature of Claim 1 | Disclosure Status |
| The method comprises oral administration of a tablet | Partially Disclosed |
| The formulation is a sustained-release hydrogel-forming formulation | Partially Disclosed |
| The formulation comprises a carrier | Fully Disclosed |
| The formulation provides continuous drug release for at least 4 hours after oral administration | Fully Disclosed |
Key Excerpt from AU6802094A
“The release of the first portion of the drug, giving the first phase of the release profile, may also take place in a sustained manner to further reduce the risk of side effects. It is preferred that the release of the first portion occurs over an extended period to enhance therapeutic efficacy.”
#3. MX2009004681A
This patent, filed on October 30, 2007, discloses a controlled-release pharmaceutical composition containing angiotensin II receptor blockers and HMG-CoA reductase inhibitors. However, this reference does not explicitly disclose treatment for overactive bladder or quantifiable food effect reduction.
Why this qualifies as Potential Related Patent?
- Oral administration of a tablet – The reference describes a tablet-based pharmaceutical composition, aligning with the oral administration feature of US12097189B1.
- Sustained-release formulation – The patent discusses delayed-release drug delivery, which partially aligns with the hydrogel-based sustained-release approach in US12097189B1.
- Use of carriers – The formulation includes enteric polymers such as polyvinyl acetate-phthalate, methacrylic acid copolymers, and hydroxypropylmethylcellulose phthalate, which serve as carriers in the controlled-release system.
- Sustained drug release for at least 4 hours – The reference specifies that drug release is delayed and extended over several hours, fulfilling the requirement for continuous drug release for at least 4 hours in US12097189B1.
- Comparative dissolution studies – The patent includes comparative dissolution tests but does not explicitly discuss reduced food effect compared to an immediate-release formulation.
Which features of US12097189B1 are disclosed in MX2009004681A?
| Key Feature of Claim 1 | Disclosure Status |
| The method comprises oral administration of a tablet | Partially Disclosed |
| The formulation is a sustained-release hydrogel-forming formulation | Partially Disclosed |
| The formulation comprises a carrier | Fully Disclosed |
| The formulation provides continuous drug release for at least 4 hours after oral administration | Fully Disclosed |
Key Excerpt from MX2009004681A
“It is the object of the present invention to provide a drug delivery system in which the release of one of the two active ingredients is individually delayed by delay time to provide pharmacological benefits, as well as a method for preparing such a system.”
#4. RU2495666C2
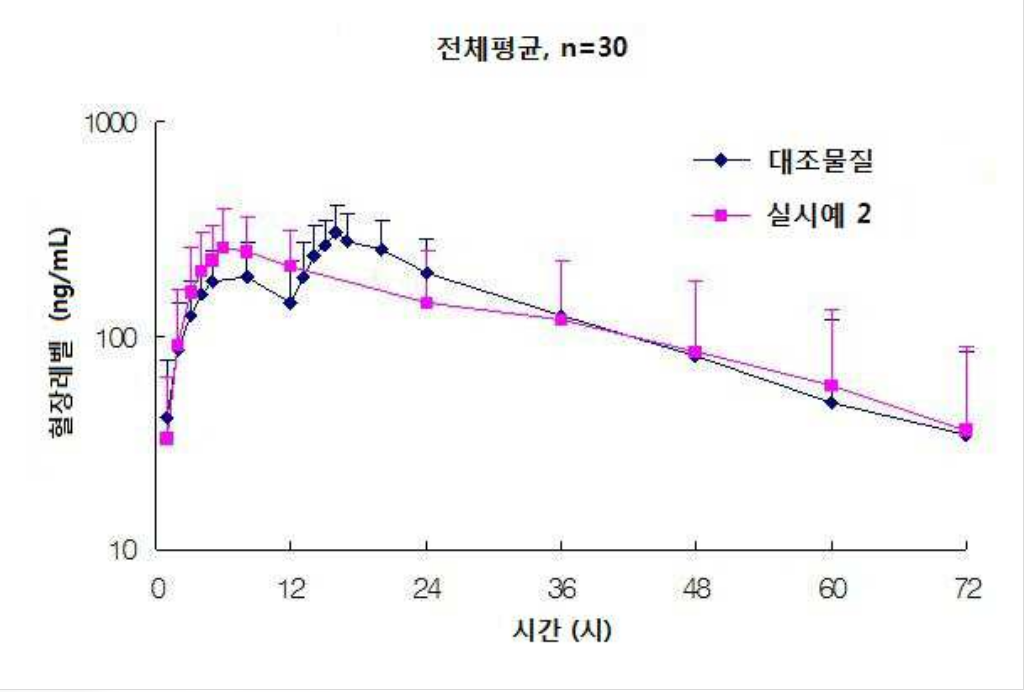
This patent, filed on September 28, 2009, discloses a modified-release pharmaceutical composition designed to treat overactive bladder while minimizing food effects on drug absorption. The formulation includes hydrogel-forming polymers to facilitate sustained drug release and enhance absorption.
Why this qualifies as Potential Related Patent?
- Method for treating overactive bladder with reduced food effect – The reference explicitly states that the pharmaceutical composition is used to treat overactive bladder and is designed to minimize the effects of food intake on drug absorption, fully aligning with US12097189B1.
- Oral administration of a tablet – The patent describes oral tablet formulations, meeting the requirement for tablet-based drug administration.
- Sustained-release hydrogel formulation – The reference details the use of hydrogel-forming polymers, ensuring sustained drug release over time.
- Continuous drug release for at least 4 hours – The invention is designed to provide controlled drug release for an extended period, fulfilling the 4-hour release requirement.
- Comparison to immediate-release formulations – The patent includes clinical comparisons showing that the modified-release formulation reduces food effects compared to immediate-release versions.
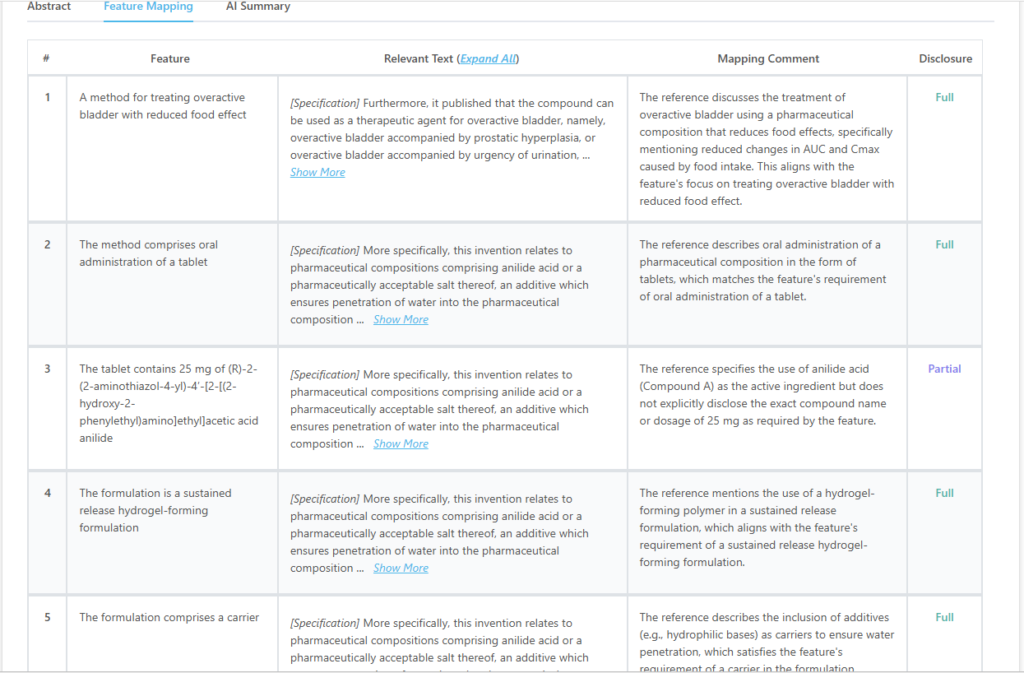
Here’s how patent feature mapping from the tool looks like:
Source: GPS
Which features of US12097189B1 are disclosed in RU2495666C2?
| Key Feature of Claim 1 | Disclosure Status |
| A method for treating overactive bladder with reduced food effect | Fully Disclosed |
| The method comprises oral administration of a tablet | Fully Disclosed |
| The tablet contains 25 mg of (R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetic acid anilide | Partially Disclosed |
| The formulation is a sustained-release hydrogel-forming formulation | Fully Disclosed |
| The formulation comprises a carrier | Fully Disclosed |
| The formulation provides continuous drug release for at least 4 hours after oral administration | Fully Disclosed |
| The reduced food effect is compared to an immediate-release capsule formulation of the same compound | Fully Disclosed |
| The reduced food effect is characterized by a difference in the rate of decrease of Cmax of 10% or more | Fully Disclosed |
Key Excerpt from RU2495666C2
“The present invention provides a pharmaceutical composition for modified release, which is not affected by the effects of diet and exhibits reduced change in AUC and Cmax, ensuring stable pharmacokinetic properties.”
#5. KR20080092885A
This patent, filed on April 14, 2008, discloses a sustained-release pharmaceutical composition containing a semi-permeable membrane for controlled drug delivery. The formulation employs excipients and carriers to maintain controlled release properties and prolong drug release duration.
Why this qualifies as Potential Related Patent?
- Oral administration of a tablet – The reference describes oral sustained-release tablets, aligning with the claimed tablet-based administration method in US12097189B1.
- Sustained-release formulation – The patent details a semi-permeable membrane-based controlled release system, which partially aligns with the hydrogel-forming sustained-release formulation in US12097189B1.
- Carrier inclusion – The composition incorporates excipients and carriers, which fulfill the requirement for a carrier in the formulation.
- Prolonged drug release – The reference states that the formulation provides continuous drug release over time, although it does not explicitly confirm a minimum of 4 hours.
- Minimized environmental effects – The patent highlights controlled drug release without external influence, indirectly suggesting reduced variability in drug absorption. However, it does not explicitly discuss food effect reduction.
Which features of US12097189B1 are disclosed in KR20080092885A?
| Key Feature of Claim 1 | Disclosure Status |
| The method comprises oral administration of a tablet | Fully Disclosed |
| The formulation is a sustained-release hydrogel-forming formulation | Partially Disclosed |
| The formulation comprises a carrier | Fully Disclosed |
| The formulation provides continuous drug release for at least 4 hours after oral administration | Partially Disclosed |
Key Excerpt from KR20080092885A
“The present invention relates to a sustained-release pharmaceutical composition capable of delivering one or more active ingredients through a semi-permeable membrane, allowing constant drug release over time, independent of external environmental factors.”
Feature Comparison Table
| Key Feature of Claim 1 | KR20130106456A | AU6802094A | MX2009004681A | RU2495666C2 | KR20080092885A |
| A method for treating overactive bladder with reduced food effect | Not Disclosed | Not Disclosed | Not Disclosed | Fully Disclosed | Not Disclosed |
| The method comprises oral administration of a tablet | Partially Disclosed | Partially Disclosed | Partially Disclosed | Fully Disclosed | Fully Disclosed |
| The tablet contains 25 mg of (R)-2-(2-aminothiazol-4-yl)-4′-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]acetic acid anilide | Not Disclosed | Not Disclosed | Not Disclosed | Partially Disclosed | Not Disclosed |
| The formulation is a sustained-release hydrogel-forming formulation | Fully Disclosed | Partially Disclosed | Partially Disclosed | Fully Disclosed | Partially Disclosed |
| The formulation comprises a carrier | Fully Disclosed | Fully Disclosed | Fully Disclosed | Fully Disclosed | Fully Disclosed |
| The formulation provides continuous drug release for at least 4 hours after oral administration | Partially Disclosed | Fully Disclosed | Fully Disclosed | Fully Disclosed | Partially Disclosed |
| The reduced food effect is compared to an immediate-release capsule formulation of the same compound | Not Disclosed | Not Disclosed | Not Disclosed | Fully Disclosed | Not Disclosed |
| The reduced food effect is characterized by a difference in rate of decrease of Cmax of 10% or more | Not Disclosed | Not Disclosed | Not Disclosed | Fully Disclosed | Not Disclosed |
Uncover Related Patents effortlessly with Global Patent Search
Navigating patent disputes requires precision, and finding the right related patent can make all the difference. With Global Patent Search, you can eliminate the guesswork and uncover critical related patent references in just a few clicks. Here is how GPS streamlines the process:
Instant smart search: Enter a patent number or key technical terms, and GPS retrieves the most relevant prior art from global databases.
Source: GPS
Advanced feature mapping: Compare patent claims side by side with existing technologies to identify key overlaps and distinctions.
Comprehensive related patent reports: Gain access to structured analyses showing how each reference aligns with the disputed patent.
Insights backed by data: Use clear, evidence-backed reports to challenge novelty and obviousness with confidence.
Stay ahead of litigation: Whether defending or challenging a patent, GPS helps legal teams and researchers make informed decisions faster.
Don’t let a crucial related patent reference slip through the cracks. With GPS, you have the power to uncover, analyze, act, and ensure the strongest possible position in any patent dispute.
Take the Guesswork Out of Related Patent Research
Patent disputes are not won on guesswork; they are won on cold, hard evidence. The right related patent can dismantle a patent claim, saving millions in litigation costs. But if you’re relying on outdated search methods, you are already behind.
With Global Patent Search (GPS), you get:
#1. Lightning-fast related patent discovery – Search by patent number, description, or technical features.
#2. Pinpoint accuracy – Feature-by-feature mapping exposes overlaps and weaknesses.
#3. Litigation-ready reports – Structured, data-backed insights at your fingertips.
#4. A tactical edge – Build a case that stands up to scrutiny.
Don’t play defense; go on the attack. Arm your patent strategy with Global Patent Search and leave no claim unchallenged.
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search (GPS) tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.