Could this Novartis patent face a challenge? The pharmaceutical giant Novartis Pharmaceuticals Corporation, alongside Astex Therapeutics Ltd., is engaged in litigation against Natco Pharma concerning Patent US9193732B2. This patent claims novel salts and methods for their synthesis and therapeutic applications.
The patent is particularly relevant in the field of oncology and kinase inhibition, as it discloses pharmaceutical compositions designed to inhibit cyclin-dependent kinases (CDK1, CDK2, CDK3, CDK4, CDK5, CDK6, and CDK9), which are critical in cancer treatment. The litigation underscores the importance of this patent in the competitive pharmaceutical market.
However, with any high-stakes pharmaceutical patent, the possibility of prior art challenges arises. Prior art references could impact the patent’s validity by demonstrating that its key claims were already disclosed before the filing date. Leveraging the Global Patent Search (GPS) tool, we analyze potential prior art references that might challenge US9193732B2.
Understanding Patent US9193732B2
The patent US9193732B2 covers the process of making 7-Cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide and its salts. It also claims novel salts of the compound, pharmaceutical compositions containing these salts, and methods of treatment using these compositions.
The key features of this patent are:
#1. Synthesis Process – A method of preparing 7-Cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide and its succinate salt.
#2. Novel Salt Forms – The succinate salt form of the compound which demonstrates stability and solubility advantages over other forms.
#3. Pharmaceutical Composition – Formulations incorporating the salt of Formula (II) with acceptable carriers, diluents, or excipients.
#4. Therapeutic Application – Treatment of diseases responsive to CDK inhibition, including various cancers such as breast cancer, lung cancer, ovarian cancer, and lymphoma.
Source: US9193732B2
The patent is critical in Novartis’ oncology portfolio, protecting the commercial viability of its CDK inhibitors. The litigation with Natco Pharma Limited likely stems from a challenge regarding the patent’s validity. It particularly concerns the novelty and non-obviousness of the succinate salt form and its synthesis process.
Evaluating prior art references that disclose similar compounds or methods is essential in assessing the strength of the patent’s claims.
Potential Prior Art References for US9193732B2
#1.IN3280DEN2014A
This Indian patent, filed on April 23, 2014, discloses macrocyclic kinase inhibitors targeting LRRK2, a key enzyme involved in neurodegenerative diseases. It describes various chemical structures, including their salt forms, potential pharmaceutical applications, and biological activity.
Why this qualifies as Potential Prior Art?
- Succinate Salt Formation – The reference explicitly discloses the succinate salt of 7-Cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide, matching the compound in F1.
- Structural Similarity – The compounds of Formula I in this reference align structurally with the claimed compound.
- Kinase Inhibitor Activity – The disclosed compounds inhibit LRRK2, similar to the mechanism described in the subject patent.
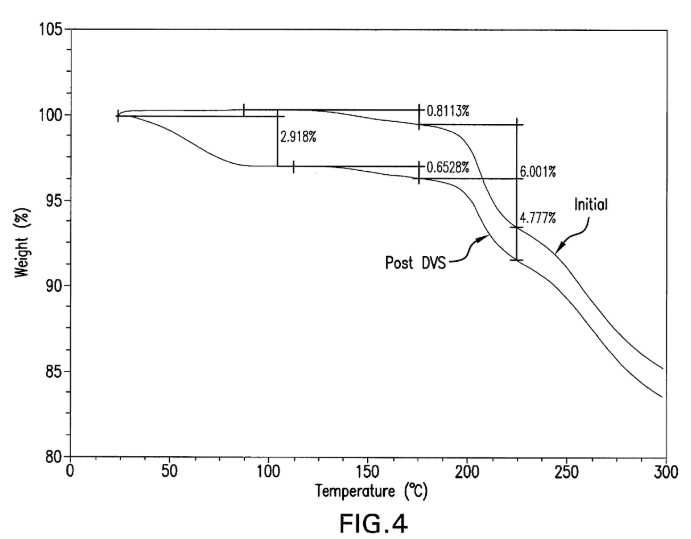
- No Explicit XRPD Data – While the reference includes figures related to cellular phosphostatus, it does not provide an X-ray Powder Diffraction (XRPD) pattern matching Figure 2.
Which Features of US9193732B2 are disclosed by IN3280DEN2014A?
| Key Feature of Claim 1 | Disclosure Status |
| Succinate salt of 7-Cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide | Fully Disclosed |
| Kinase inhibitor activity | Fully Disclosed |
| Structural similarity to the claimed compound | Fully Disclosed |
Key Excerpt from IN3280DEN2014A:
“The reference discloses compounds of Formula I, which include the succinate salt of 7-Cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide. Paragraph [0042] and [0273] describe the structural elements and salt forms, which align with the feature F1.”
#2. CA2345809A1
This patent, filed on Oct. 13, 1999, describes a class of 6-substituted pyrazolo[3,4-d]pyrimidin-4-one compounds designed for use as cyclin-dependent kinase (CDK) inhibitors. The compounds are explored for their pharmaceutical formulations, including potential solid and liquid dosage forms for administration.
Why this qualifies as Potential Prior Art?
- Structural Similarities to Pyrazolopyrimidine Derivatives – The reference discloses a broad class of pyrazolo[3,4-d]pyrimidin-4-one compounds, some of which share structural similarities with the claimed succinate salt of 7-Cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide. However, the exact compound is not explicitly disclosed.
- Pharmaceutical Formulations – The patent details various pharmaceutical compositions containing the active ingredient, including oral solid forms (capsules, tablets, powders) and liquid forms (elixirs, syrups, suspensions). This aligns with the subject patent’s focus on pharmaceutical applications.
- Potential for Cyclin-Dependent Kinase Inhibition – The compounds in this reference are designed as CDK inhibitors, which is a shared functional objective with the claimed invention, suggesting a possible overlap in intended therapeutic use.
- Lack of Explicit XRPD Data – The reference does not provide any X-ray Powder Diffraction (XRPD) data or patterns, which is a key distinguishing feature of the subject patent.
Which Features of US9193732B2 are disclosed by CA2345809A1?
| Key Feature of Claim 1 | Disclosure Status |
| Pharmaceutical formulations (oral, solid, liquid) | Fully Disclosed |
| Cyclin-dependent kinase inhibition | Fully Disclosed |
Key Excerpt from CA2345809A1:
“Dosage forms of compositions suitable for administration contain from about 1 mg to about 100 mg of active ingredient per unit. In these pharmaceutical compositions, the active ingredient will ordinarily be present in an amount of about 0.5-95% by weight based on the total weight of the composition. The active ingredient can be administered orally in solid dosage forms, such as capsules, tablets, and powders, or in liquid dosage forms, such as elixirs, syrups, and suspensions. It can also be administered parenterally, in sterile liquid dosage forms.”
#3. KR20120097473A
This patent, filed on July 2, 2010, discloses pyrazolopyrimidine-based Janus kinase (JAK) inhibitor compounds, including their pharmaceutical formulations and therapeutic applications. The reference describes compounds of Formula (I), which encompass various derivatives and salts with potential JAK inhibitory properties.
Why this qualifies as Potential Prior Art?
- Structural Overlap with Pyrazolopyrimidine Derivatives – The reference describes compounds of Formula (I), which include various substituents and derivatives. While the exact compound, a succinate salt of 7-Cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide, is not explicitly disclosed, the structural framework of pyrazolopyrimidine derivatives is similar.
- JAK Inhibition as a Therapeutic Target – The reference specifies that the disclosed compounds are inhibitors of Janus kinases, suggesting functional relevance to kinase inhibition, which overlaps with the subject patent’s application.
- Pharmaceutical Formulations – The patent covers pharmaceutical formulations containing these compounds, which can be used in treating diseases responsive to JAK inhibition, showing a shared therapeutic focus.
- Lack of Explicit XRPD Data – The reference does not provide any mention of X-ray Powder Diffraction (XRPD) patterns, which is a distinguishing feature of the subject patent.
Which Features of US9193732B2 are disclosed by KR20120097473A?
| Key Feature of Claim 1 | Disclosure Status |
| Succinate salt of 7-Cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide | Partially Disclosed |
| JAK inhibition as a therapeutic mechanism | Fully Disclosed |
| Pharmaceutical formulations (oral, solid, liquid) | Fully Disclosed |
Key Excerpt from KR20120097473A:
“In one embodiment, compounds of formula (I) and pharmaceutical formulations thereof are provided for the treatment of diseases, conditions, and/or disorders responsive to inhibition of one or more Janus kinases. Another embodiment includes a compound of Formula (I): an enantiomer, diastereomer, or pharmaceutically acceptable salt thereof.”
#4. BR112014007622A2
This patent, filed on Sept. 28, 2012, describes macrocyclic inhibitors targeting FLT3 kinase. It includes compounds of Formula I and their pharmaceutical applications. The compounds are presented with various stereoisomers, salts, and solvates, making them relevant to kinase inhibition for therapeutic use.
Why this qualifies as Potential Prior Art?
- Structural Similarities with Pyrazolopyrimidine Compounds – The reference describes a broad class of kinase inhibitors, including derivatives with substituents similar to pyrazolopyrimidine structures. While the specific compound, a succinate salt of 7-Cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide, is not explicitly disclosed, the reference contains structural flexibility.
- FLT3 Kinase Inhibition Mechanism – The disclosed compounds target FLT3 kinase, a mechanism relevant to kinase inhibitors, which overlaps with the function of the subject patent’s compounds.
- Pharmaceutical Formulation and Administration – The patent describes the use of these inhibitors in different pharmaceutical formulations, including solid and liquid dosage forms, for treating cancers and other FLT3-related diseases.
- Absence of XRPD Data – The reference does not provide any mention of X-ray Powder Diffraction (XRPD) data or figures, which differentiates it from the subject patent.
Which Features of US9193732B2 are disclosed by BR112014007622A2?
| Key Feature of Claim 1 | Disclosure Status |
| FLT3 kinase inhibition as a therapeutic mechanism | Fully Disclosed |
| Pharmaceutical formulations (oral, solid, liquid) | Fully Disclosed |
Key Excerpt from BR112014007622A2:
“In a first aspect, the present invention provides a compound of Formula I or a stereoisomer, tautomer, racemic, metabolite, pro- or predrug, salt, hydrate, N-oxide form, or solvate thereof. The compounds described are useful as inhibitors of FLT3 kinase, showing potential for therapeutic applications in oncology.”
#5. AU2003251010A1
This patent, filed on July 8, 2003, discloses 3-furanyl derivatives of toxoflavine as kinase inhibitors. It discusses their pharmaceutical compositions, methods of preparation, and potential therapeutic applications. The reference also includes various salt forms of the compounds, including succinate salts.
Why this qualifies as Potential Prior Art?
- Succinate Salt Formation – The reference explicitly mentions the formation of pharmaceutically acceptable salts, including succinate salts, though it does not specify the exact compound in question.
- Kinase Inhibitor Activity – The disclosed compounds function as kinase inhibitors, which is relevant to the mechanism of the subject patent.
- Pharmaceutical Formulations and Solubility Enhancements – The reference details various formulation methods, including salt forms, for improving drug solubility and stability.
- No Explicit XRPD Data – While the reference mentions X-ray diffraction techniques for characterization, it does not provide an explicit X-ray Powder Diffraction (XRPD) pattern matching Figure 2.
Which Features of US9193732B2 are disclosed by AU2003251010A1?
| Key Feature of Claim 1 | Disclosure Status |
| Kinase inhibitor activity | Fully Disclosed |
| Pharmaceutical formulations including salt forms | Fully Disclosed |
Key Excerpt from AU2003251010A1:
“The pharmaceutically acceptable addition salts as mentioned hereinabove are meant to comprise the therapeutically active non-toxic acid addition salt forms which the compounds of formula (I) are able to form. The latter can conveniently be obtained by treating the base form with such appropriate acid. Appropriate acids comprise, for example, inorganic acids such as hydrohalic acids, e.g., hydrochloric or hydrobromic acid; sulfuric; nitric; phosphoric and the like acids; or organic acids such as, for example, acetic, propanoic, hydroxyacetic, lactic, pyruvic, oxalic, malonic, succinic.”
Feature Comparison Table
| Feature | IN3280DEN2014A | CA2345809A1 | KR20120097473A | BR112014007622A2 | AU2003251010A1 |
| Succinate salt of 7-Cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid dimethylamide | Fully Disclosed | Not Disclosed | Partially Disclosed | Not Disclosed | Not Disclosed |
| Kinase inhibitor activity | Fully Disclosed | Fully Disclosed | Fully Disclosed | Fully Disclosed | Fully Disclosed |
| Structural similarity to the claimed compound | Fully Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed |
| X-ray Powder Diffraction (XRPD) pattern | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
How to Find Prior Art Using Global Patent Search
In the high-stakes world of patent litigation, finding the right prior art can decide between upholding a patent or seeing it invalidated. The Global Patent Search (GPS) tool is designed to cut through the noise and deliver relevant prior art with speed and precision. Here’s how it simplifies the process:
Search smarter – Enter a patent number (e.g., US9193732B2) or key technical terms, and GPS instantly retrieves relevant prior art references. No more sifting through thousands of unrelated documents.
Source: GPS
Feature-to-Feature Mapping – GPS doesn’t just list similar patents—it analyzes key technical features and maps them against existing disclosures, highlighting overlaps and gaps.
Curated Prior art reports – Access a refined selection of prior art references that matter most, complete with feature mapping analysis and direct excerpts from patent documents.
Make informed decisions – Whether challenging or defending a patent, having the right prior art at your fingertips is essential for building a winning strategy.
Patent research does not have to be tedious. With Global Patent Search, you get instant access to the most relevant prior art, empowering you to act confidently.
Take the Guesswork Out of Prior Art Research
Patent disputes can be complex, but uncovering the right prior art doesn’t have to be. The Global Patent Search (GPS) tool provides:
#1. Instant, targeted results – No more endless searching. GPS finds the most relevant prior art in seconds.
#2. Accurate feature mapping – Compare patent claims against existing disclosures with clarity.
#3. Data-driven analysis – Strengthen your case with verifiable, high-impact prior art references.
Do not leave your litigation strategy to chance. Ensure you have the best prior art at your fingertips. Start your search with Global Patent Search today!
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The prior art references mentioned are preliminary results from the Global Patent Search (GPS) tool and do not guarantee legal significance. For a comprehensive prior art analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.