The patent showdown between Bristol-Myers Squibb, Pfizer, and Umedica Laboratories Pvt. Ltd. has put patent US9326945B2 in the hot seat. Covering a precisely formulated version of apixaban, this patent claims to enhance bioavailability through controlled particle sizing. This is an innovation critical to its pharmaceutical success.
Touted as a breakthrough for consistent drug absorption and efficacy, this formulation has been a cornerstone for its patent holders. But is it truly groundbreaking, or does related patent threaten its exclusivity? If earlier patents reveal similar techniques for particle size optimization and bioequivalence, the foundation of US9326945B2 could be in jeopardy.
This is where Global Patent Search (GPS) steps in. It brings potential related patents to light and challenges the patent’s validity. Before diving into the related patents that could shake things up, let us first break down what this patent protects.
Understanding Patent US9326945B2
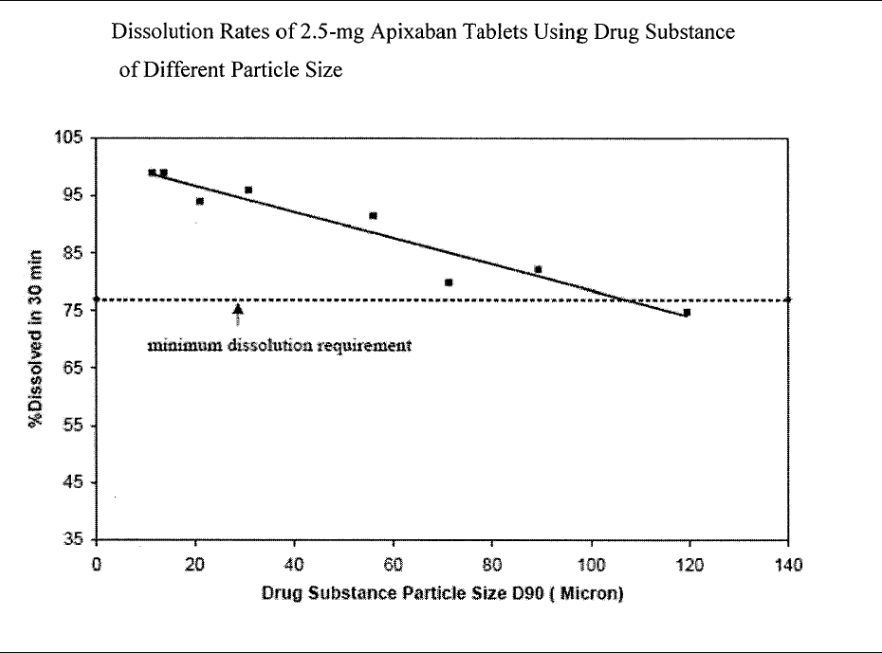
Bristol-Myers Squibb and Pfizer own US9326945B2 and cover pharmaceutical compositions of apixaban, a Factor Xa inhibitor used to prevent and treat thromboembolic disorders. The patent focuses on crystalline apixaban particles with a controlled particle size (D90 ≤ 89 μm) to enhance bioavailability and ensure consistent therapeutic effects. The formulation improves dissolution rates and absorption, particularly through dry granulation techniques.
Source: Google Patents
Here are its key features:
#1. Crystalline apixaban particles – The formulation uses finely controlled apixaban particles, with a D90 size of ≤ 89 μm, to enhance bioequivalence.
#2. Optimized dissolution rate – The composition is designed to dissolve at least 77% within 30 minutes in a pH 6.8 phosphate buffer.
#3. Dry granulation manufacturing – The patent claims a dry granulation process for consistent particle size distribution, ensuring uniform drug absorption.
#4. Surfactant inclusion – The formulation may contain 1–2% sodium lauryl sulfate to improve wetting and enhance dissolution.
The patent is currently being challenged by Umedica Laboratories Pvt. Ltd., which is disputing its validity. The central issue is whether US9326945B2’s claims on particle size optimization and bioequivalence are genuinely novel or if prior formulations have already disclosed similar techniques. If earlier references describe particle size control in apixaban or other Factor Xa inhibitors, the exclusivity of this patent could be at serious risk.
Potential Related Patent References for US9326945B2
#1. EA201000064A1
This patent, filed on June 27, 2008, describes new anticoagulant compounds and pharmaceutical compositions for treating thrombotic conditions. The invention covers solid oral dosage forms, such as tablets or capsules, that contain pharmaceutically acceptable carriers and a therapeutically effective amount of an anticoagulant.
Why this qualifies as Potential Related Patent?
- Solid pharmaceutical composition – The reference describes tablets and capsules for oral administration, aligning with the formulation type in US9326945B2.
- Pharmaceutically acceptable carriers – It mentions binding agents and excipients, which match the use of carriers in US9326945B2.
- Therapeutically effective amount of anticoagulant – The reference discloses anticoagulant compositions designed for thrombotic condition treatment, partially aligning with the claimed use of apixaban.
Which features of US9326945B2 are disclosed by EA201000064A1?
| Key Feature of Claim 1 | Disclosure Status |
| The composition is a solid pharmaceutical composition | Fully Disclosed |
| The composition comprises a pharmaceutically acceptable diluent or carrier | Fully Disclosed |
| The composition contains a therapeutically effective amount of apixaban | Partially Disclosed |
Key Excerpt from EA201000064A1:
“For oral administration, the pharmaceutical compositions can be, for example, tablets or capsules with pharmaceutically acceptable additives, such as binding agents (for example, peptized maize starch, polyvinylpyrrolidinone)…”
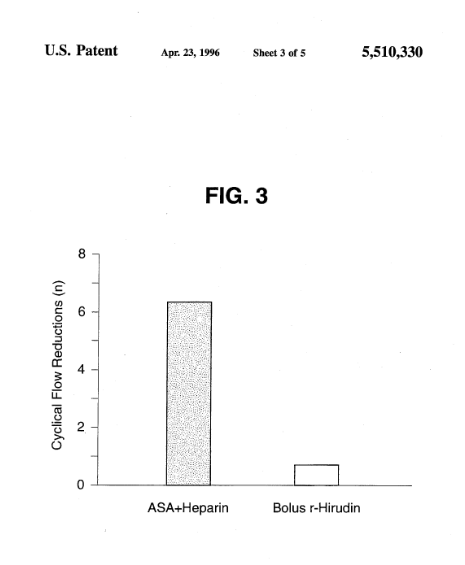
#2. US5510330A
This patent, filed on March 25, 1994, describes combinations of thrombolytically active proteins and non-heparin anticoagulants for the treatment of thrombotic diseases. It discloses pharmaceutical compositions containing anticoagulants that act independently of heparin, but its primary focus is on intravenous administration rather than solid formulations.
Source: GPS
Why this qualifies as Potential Related Patent?
- Pharmaceutical compositions for thrombotic disease treatment – The reference discusses the use of anticoagulants and thrombolytic agents, aligning with the therapeutic objective of US9326945B2.
- Non-heparin anticoagulants – The patent includes direct thrombin inhibitors and Factor Xa inhibitors, which could overlap with apixaban’s mechanism of action.
- Pharmaceutical carriers – The reference suggests that compositions may contain carriers or diluents, though it does not specify solid formulations similar to US9326945B2.
Which features of US9326945B2 are disclosed by US5510330A?
| Key Feature of Claim 1 | Disclosure Status |
| The composition comprises a pharmaceutically acceptable diluent or carrier | Partially Disclosed |
| The composition comprises a pharmaceutically acceptable diluent or carrier | Partially Disclosed |
Key Excerpt from US5510330A:
“The present invention provides pharmaceutical compositions and methods for the treatment of a patient with a thrombotic disease. According to the invention, the pharmaceutical compositions may include direct thrombin inhibitors and other anticoagulants that function independently of heparin.”
#3. US20040053408A1
This patent, filed on July 1, 2003, describes compositions and methods for selective dissolution of nascent intravascular blood clots. The invention covers therapeutic agents, such as anticoagulants, coupled to carriers designed to prevent deep penetration into existing clots, ensuring targeted action for thrombolytic therapy.
Why this qualifies as Potential Related Patent?
- Pharmaceutical compositions with therapeutic agents – The reference discusses formulations that include anticoagulants, aligning with the therapeutic focus of US9326945B2.
- Use of carriers for drug delivery – The patent discloses carriers for controlled drug release, which could be relevant in assessing the formulation strategy of the contested patent.
- Potential inclusion of pharmaceutically acceptable carriers – The reference describes therapeutic agents biocompatibly coupled to carriers, which may suggest the use of acceptable diluents or excipients.
Which features of US9326945B2 are disclosed by US20040053408A1?
| Key Feature of Claim 1 | Disclosure Status |
| The composition is a solid pharmaceutical composition | Partially Disclosed |
| The composition comprises a pharmaceutically acceptable diluent or carrier | Partially Disclosed |
| The composition contains a therapeutically effective amount of apixaban | Partially Disclosed |
Key Excerpt from US20040053408A1:
“Accordingly, the present invention relates to compositions comprising a therapeutic agent, such as an anti-thrombotic agent, biocompatibly coupled to a carrier sized to inhibit penetration into existing clots, such as a red blood clot.”
#4. KR20120106990A
This patent, filed on January 5, 2011, describes anti-heparin compounds for antagonizing the effects of heparin-based anticoagulants. The invention focuses on compositions containing pharmaceutically acceptable salts and carriers, which may be relevant in evaluating prior formulations of anticoagulants.
Why this qualifies as Potential Related Patent?
- Pharmaceutical compositions for anticoagulation therapy – The reference discusses compositions for counteracting heparin, which aligns with the broader anticoagulant function of US9326945B2.
- Use of pharmaceutically acceptable carriers – The patent suggests that compositions may include pharmaceutically acceptable salts and carriers, potentially aligning with formulation claims of the contested patent.
- Solid pharmaceutical compositions – The reference discusses solid formulations in the context of pharmaceutical compositions, though it does not explicitly define them as solid dosage forms like tablets.
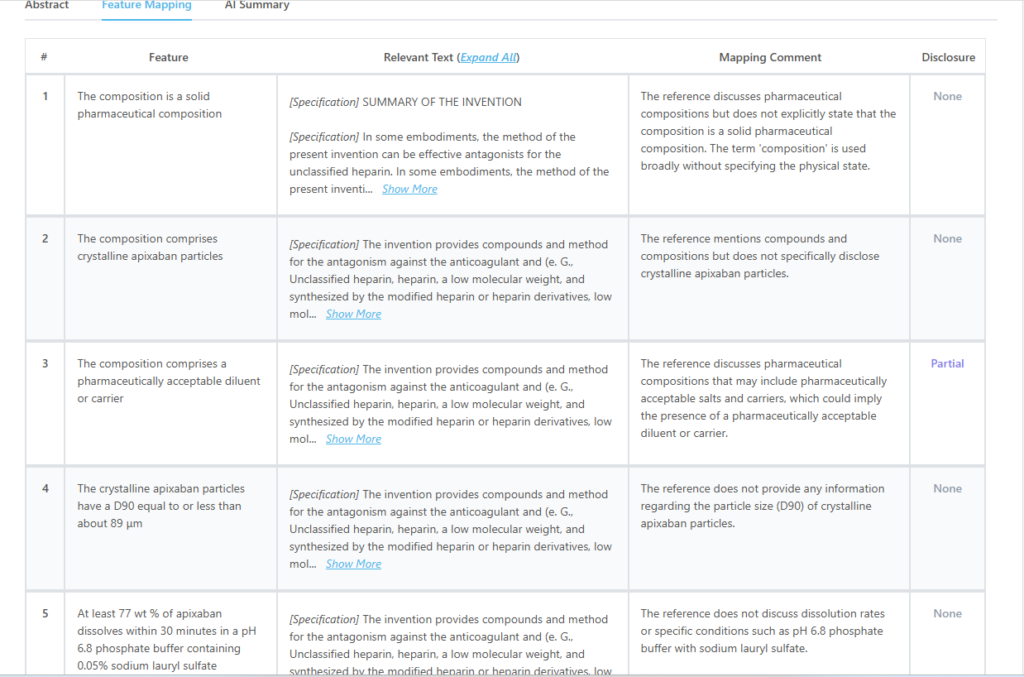
Here’s what the mapping from the tool for this particular patent looks like:
Source: GPS
Which features of US9326945B2 are disclosed by KR20120106990A?
| Key Feature of Claim 1 | Disclosure Status |
| The composition is a solid pharmaceutical composition | Partially Disclosed |
| The composition comprises a pharmaceutically acceptable diluent or carrier | Partially Disclosed |
| The composition contains a therapeutically effective amount of apixaban | Partially Disclosed |
Key Excerpt from KR20120106990A:
“The invention provides compounds and methods for antagonizing anticoagulants, including pharmaceutical compositions comprising pharmaceutically acceptable salts and carriers.”
#5. WO2011027175A1
This patent, filed on September 6, 2010, describes antithrombotic compounds and pharmaceutical compositions designed for preventing and treating thrombotic conditions. The invention covers various anticoagulants formulated with pharmaceutically acceptable excipients, though it does not explicitly mention apixaban.
Why this qualifies as Potential Related Patent?
- Pharmaceutical compositions for antithrombotic therapy – The reference describes compositions containing anticoagulant compounds, aligning with the therapeutic objective of US9326945B2.
- Use of pharmaceutically acceptable carriers – The patent specifies that pharmaceutical compositions may contain excipients, which could include diluents or carriers, potentially relevant to formulation claims of US9326945B2.
- Solid pharmaceutical compositions – The reference discusses solid forms of pharmaceutical compositions, though it does not explicitly confirm that all formulations are solid dosage forms.
Which features of US9326945B2 are disclosed by WO2011027175A1?
| Key Feature of Claim 1 | Disclosure Status |
| The composition is a solid pharmaceutical composition | Partially Disclosed |
| The composition comprises a pharmaceutically acceptable diluent or carrier | Partially Disclosed |
| The composition contains a therapeutically effective amount of apixaban | Partially Disclosed |
Key Excerpt from WO2011027175A1:
“The present invention also provides a pharmaceutical composition comprising the compound described above and one or more pharmaceutically acceptable excipients. Suitable pharmaceutical excipients are well known to those skilled in the art.”
Feature Comparison Table
| Key Feature of Claim 1 | EA201000064A1 | US5510330A | US20040053408A1 | KR20120106990A | WO2011027175A1 |
| The composition is a solid pharmaceutical composition | Fully Disclosed | Not Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed |
| The composition comprises crystalline apixaban particles | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| The composition comprises a pharmaceutically acceptable diluent or carrier | Fully Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed |
| The crystalline apixaban particles have a D90 equal to or less than about 89 μm | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| At least 77 wt % of apixaban dissolves within 30 minutes in a pH 6.8 phosphate buffer containing 0.05% sodium lauryl sulfate | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| The composition contains a therapeutically effective amount of apixaban | Partially Disclosed | Not Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed |
| The composition is a solid pharmaceutical composition | Fully Disclosed | Not Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed |
| The composition comprises crystalline apixaban particles | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| The composition comprises a pharmaceutically acceptable diluent or carrier | Fully Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed |
| The crystalline apixaban particles have a D90 equal to or less than about 89 μm | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| At least 77 wt % of apixaban dissolves within 30 minutes in a pH 6.8 phosphate buffer containing 0.05% sodium lauryl sulfate | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed | Not Disclosed |
| The composition contains a therapeutically effective amount of apixaban | Partially Disclosed | Not Disclosed | Partially Disclosed | Partially Disclosed | Partially Disclosed |
How to Find Related Patents Using Global Patent Search?
When it comes to evaluating a patent’s validity, uncovering related patents is essential. The Global Patent Search (GPS) tool takes the guesswork out of this process, offering a fast, data-backed approach to identifying relevant references. Using this tool, you can:
Search by patent number or description: Simply enter US9326945B2 or related keywords to generate a list of potentially relevant related patents.
Utilize feature mapping: The GPS tool automatically analyzes and compares key features of the subject patent against existing patents, identifying areas of overlap.
Review matching related patent: Get instant access to a curated selection of related patent references, complete with detailed feature mapping and direct excerpts.
Analyze the details: See at a glance which features are fully disclosed, partially disclosed, or not disclosed in each reference, making assessment easier.
Make informed decisions: Whether you are defending or challenging a patent, data-backed insights from GPS help refine legal strategies with confidence.
By transforming the complex task of related patent research into a structured, efficient process, Global Patent Search ensures you get accurate results without the manual legwork.
Take the Guesswork Out of Related Patent Research
Patent battles are high-stakes, but your related patent search does not have to be. The Global Patent Search tool cuts through the noise, delivering:
- Instant results – Skip the endless digging and get AI-powered insights in seconds.
- Accurate feature mapping – See exactly where patents and related patent overlap.
- Data-backed insights – Build a rock-solid case with structured and verifiable analysis.
When defending or challenging a patent, the right related patent can make all the difference. Find it faster with Global Patent Search.
Disclaimer: The information provided in this article is for informational purposes only and should not be considered legal advice. The related patent references mentioned are preliminary results from the Global Patent Search (GPS) tool and do not guarantee legal significance. For a comprehensive related patent analysis, we recommend conducting a detailed search using GPS or consulting a patent attorney.